Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial
- PMID: 20215360
- PMCID: PMC2835853
- DOI: 10.1136/bmj.c1101
Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial
Abstract
Objective: To investigate the effect of vitamin A supplementation and BCG vaccination at birth in low birthweight neonates.
Design: Randomised, placebo controlled, two by two factorial trial.
Setting: Bissau, Guinea-Bissau.
Participants: 1717 low birthweight neonates born at the national hospital.
Intervention: Neonates who weighed less than 2.5 kg were randomly assigned to 25 000 IU vitamin A or placebo, as well as to early BCG vaccine or the usual late BCG vaccine, and were followed until age 12 months.
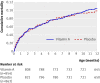
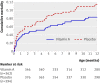
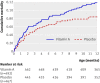
Main outcome measure: Mortality, calculated as mortality rate ratios (MRRs), after follow-up to 12 months of age for infants who received vitamin A supplementation compared with those who received placebo.
Results: No interaction was observed between vitamin A supplementation and BCG vaccine allocation (P=0.73). Vitamin A supplementation at birth was not significantly associated with mortality: the MRR of vitamin A supplementation compared with placebo, controlled for randomisation to "early BCG" versus "no early BCG" was 1.08 (95% CI 0.79 to 1.47). Stratification by sex revealed a significant interaction between vitamin A supplementation and sex (P=0.046), the MRR of vitamin A supplementation being 0.74 (95% CI 0.45 to 1.22) in boys and 1.42 (95% CI 0.94 to 2.15) in girls. When these data were combined with data from a complementary trial among normal birthweight neonates in Guinea-Bissau, the combined estimate of the effect of neonatal vitamin A supplementation on mortality was 1.08 (95% CI 0.87 to 1.33); 0.80 (95% CI 0.58 to 1.10) in boys and 1.41 (95% CI 1.04 to 1.90) in girls (P=0.01 for interaction between neonatal vitamin A and sex).
Conclusions: The combined results of this trial and the complementary trial among normal birthweight neonates have now shown that, overall, it would not be beneficial to implement a neonatal vitamin A supplementation policy in Guinea-Bissau. Worryingly, the trials show that vitamin A supplementation at birth can be harmful in girls. Previous studies and future trials should investigate the possibility that vitamin A supplementation has sex differential effects. Trial registration ClinicalTrials.gov NCT00168610.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
Figures
Comment in
-
Vitamin A supplements and survival in children.BMJ. 2010 Mar 9;340:c977. doi: 10.1136/bmj.c977. BMJ. 2010. PMID: 20215364 No abstract available.
References
-
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality: a meta-analysis. JAMA 1993;269:898-903. - PubMed
-
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. J Pediatr 1996;128:489-96. - PubMed
-
- Klemm RD, Labrique AB, Christian P, Rashid M, Shamim AA, Katz J, et al. Newborn vitamin A supplementation reduced infant mortality in rural Bangladesh. Pediatrics 2008;122:e242-e50. - PubMed
-
- Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr 2005;81:454-60. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
