Genome-wide analysis of YY2 versus YY1 target genes
- PMID: 20215434
- PMCID: PMC2896514
- DOI: 10.1093/nar/gkq112
Genome-wide analysis of YY2 versus YY1 target genes
Abstract
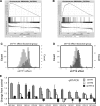
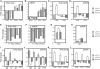
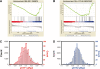
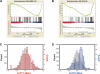
Yin Yang 1 (YY1) is a critical transcription factor controlling cell proliferation, development and DNA damage responses. Retrotranspositions have independently generated additional YY family members in multiple species. Although Drosophila YY1 [pleiohomeotic (Pho)] and its homolog [pleiohomeotic-like (Phol)] redundantly control homeotic gene expression, the regulatory contributions of YY1-homologs have not yet been examined in other species. Indeed, targets for the mammalian YY1 homolog YY2 are completely unknown. Using gene set enrichment analysis, we found that lentiviral constructs containing short hairpin loop inhibitory RNAs for human YY1 (shYY1) and its homolog YY2 (shYY2) caused significant changes in both shared and distinguishable gene sets in human cells. Ribosomal protein genes were the most significant gene set upregulated by both shYY1 and shYY2, although combined shYY1/2 knock downs were not additive. In contrast, shYY2 reversed the anti-proliferative effects of shYY1, and shYY2 particularly altered UV damage response, platelet-specific and mitochondrial function genes. We found that decreases in YY1 or YY2 caused inverse changes in UV sensitivity, and that their combined loss reversed their respective individual effects. Our studies show that human YY2 is not redundant to YY1, and YY2 is a significant regulator of genes previously identified as uniquely responding to YY1.
Figures



Similar articles
-
Biological roles of Yin Yang 2: Its implications in physiological and pathological events.J Cell Mol Med. 2020 Nov;24(22):12886-12899. doi: 10.1111/jcmm.15919. Epub 2020 Sep 23. J Cell Mol Med. 2020. PMID: 32969187 Free PMC article. Review.
-
Yin-Yang 1 and Yin-Yang 2 exert opposing effects on the promoter activity of interleukin 4.Arch Pharm Res. 2016 Apr;39(4):547-554. doi: 10.1007/s12272-015-0622-7. Epub 2015 Sep 7. Arch Pharm Res. 2016. PMID: 26345265
-
In Vivo Chromatin Targets of the Transcription Factor Yin Yang 2 in Trophoblast Stem Cells.PLoS One. 2016 May 18;11(5):e0154268. doi: 10.1371/journal.pone.0154268. eCollection 2016. PLoS One. 2016. PMID: 27191592 Free PMC article.
-
The Forgotten YY2 in Reported YY1 Expression Levels in Human Cancers.Crit Rev Oncog. 2017;22(1-2):63-73. doi: 10.1615/CritRevOncog.2017020475. Crit Rev Oncog. 2017. PMID: 29604937 Review.
-
The transcription factor YY2 has less momentous properties of an intrinsically disordered protein than its paralog YY1.FEBS Lett. 2019 Jul;593(14):1787-1798. doi: 10.1002/1873-3468.13457. Epub 2019 Jun 7. FEBS Lett. 2019. PMID: 31131888
Cited by
-
Biological roles of Yin Yang 2: Its implications in physiological and pathological events.J Cell Mol Med. 2020 Nov;24(22):12886-12899. doi: 10.1111/jcmm.15919. Epub 2020 Sep 23. J Cell Mol Med. 2020. PMID: 32969187 Free PMC article. Review.
-
YY2/BUB3 Axis promotes SAC Hyperactivation and Inhibits Colorectal Cancer Progression via Regulating Chromosomal Instability.Adv Sci (Weinh). 2024 Jul;11(26):e2308690. doi: 10.1002/advs.202308690. Epub 2024 Apr 29. Adv Sci (Weinh). 2024. PMID: 38682484 Free PMC article.
-
Characterization of interaction between Trim28 and YY1 in silencing proviral DNA of Moloney murine leukemia virus.Virology. 2018 Mar;516:165-175. doi: 10.1016/j.virol.2018.01.012. Virology. 2018. PMID: 29407374 Free PMC article.
-
Thymopentin alleviates premature ovarian failure in mice by activating YY2/Lin28A and inhibiting the expression of let-7 family microRNAs.Cell Prolif. 2021 Aug;54(8):e13089. doi: 10.1111/cpr.13089. Epub 2021 Jun 28. Cell Prolif. 2021. PMID: 34180104 Free PMC article.
-
Yy1 regulates Senp1 contributing to AMPA receptor GluR1 expression following neuronal depolarization.J Biomed Sci. 2019 Oct 20;26(1):79. doi: 10.1186/s12929-019-0582-1. J Biomed Sci. 2019. PMID: 31629407 Free PMC article.
References
-
- Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. - PubMed
-
- Kohler C, Villar CB. Programming of gene expression by Polycomb group proteins. Trends Cell. Biol. 2008;18:236–243. - PubMed
-
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. - PubMed
-
- Hormaeche I, Licht JD. Chromatin modulation by oncogenic transcription factors: new complexity, new therapeutic targets. Cancer Cell. 2007;11:475–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

