Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial
- PMID: 20215457
- PMCID: PMC2875432
- DOI: 10.2337/dc09-1481
Evaluation of an algorithm to guide patients with type 1 diabetes treated with continuous subcutaneous insulin infusion on how to respond to real-time continuous glucose levels: a randomized controlled trial
Erratum in
- Diabetes Care. 2010 Aug;33(8):1911
Abstract
Objective: To evaluate an algorithm guiding responses of continuous subcutaneous insulin infusion (CSII)-treated type 1 diabetic patients using real-time continuous glucose monitoring (RT-CGM).
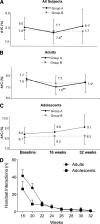
Research design and methods: Sixty CSII-treated type 1 diabetic participants (aged 13-70 years, including adult and adolescent subgroups, with A1C <or=9.5%) were randomized in age-, sex-, and A1C-matched pairs. Phase 1 was an open 16-week multicenter randomized controlled trial. Group A was treated with CSII/RT-CGM with the algorithm, and group B was treated with CSII/RT-CGM without the algorithm. The primary outcome was the difference in time in target (4-10 mmol/l) glucose range on 6-day masked CGM. Secondary outcomes were differences in A1C, low (<or=3.9 mmol/l) glucose CGM time, and glycemic variability. Phase 2 was the week 16-32 follow-up. Group A was returned to usual care, and group B was provided with the algorithm. Glycemia parameters were as above. Comparisons were made between baseline and 16 weeks and 32 weeks.
Results: In phase 1, after withdrawals 29 of 30 subjects were left in group A and 28 of 30 subjects were left in group B. The change in target glucose time did not differ between groups. A1C fell (mean 7.9% [95% CI 7.7-8.2to 7.6% [7.2-8.0]; P < 0.03) in group A but not in group B (7.8% [7.5-8.1] to 7.7 [7.3-8.0]; NS) with no difference between groups. More subjects in group A achieved A1C <or=7% than those in group B (2 of 29 to 14 of 29 vs. 4 of 28 to 7 of 28; P = 0.015). In phase 2, one participant was lost from each group. In group A, A1C returned to baseline with RT-CGM discontinuation but did not change in group B, who continued RT-CGM with addition of the algorithm.
Conclusions: Early but not late algorithm provision to type 1 diabetic patients using CSII/RT-CGM did not increase the target glucose time but increased achievement of A1C <or=7%. Upon RT-CGM cessation, A1C returned to baseline.
Figures

References
-
- Mastrototaro JJ, Cooper KW, Soundararajan G, Sanders JB, Shah RV: Clinical experience with an integrated continuous glucose sensor/insulin pump platform: a feasibility study. Adv Ther 2006;23:725–732 - PubMed
-
- Mastrototaro JJ, Soundararajan G, Cooper K, Shah R: Accuracy of real time continuous glucose monitoring in the Minimed Paradigm system (Abstract): Diabetes 2007;56(Suppl. 1):0422-P
-
- Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF: Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes 1970;19:644–655 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

