DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks
- PMID: 20215461
- PMCID: PMC2875434
- DOI: 10.2337/dc09-1914
DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks
Abstract
Objective: In the Diabetes Therapy Utilization: Researching Changes in A1C, Weight and Other Factors Through Intervention with Exenatide Once Weekly (DURATION-1) study, the safety and efficacy of 30 weeks of treatment with the glucagon-like peptide-1 receptor agonist exenatide once weekly (exenatide QW; 2 mg) was compared with exenatide BID in 295 patients with type 2 diabetes. We now report the safety and efficacy of exenatide QW in 1) patients who continued treatment for an additional 22 weeks (52 weeks total) and 2) patients who switched from exenatide BID to exenatide QW after 30 weeks.
Research design and methods: In this randomized, multicenter, comparator-controlled, open-label trial, 258 patients entered the 22-week open-ended assessment phase (n = 128 QW-only; n = 130 BID-->QW). A1C, fasting plasma glucose (FPG), body weight, blood pressure, fasting lipids, safety, and tolerability were assessed.
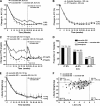
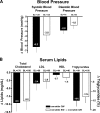
Results: Patients continuing exenatide QW maintained A1C improvements through 52 weeks (least squares mean -2.0% [95% CI -2.1 to -1.8%]). Patients switching from exenatide BID to exenatide QW achieved further A1C improvements; both groups exhibited the same A1C reduction and mean A1C (6.6%) at week 52. At week 52, 71 and 54% of all patients achieved A1C <7.0% and <or=6.5%, respectively. In both treatment arms, FPG was reduced by >40 mg/dl, and body weight was reduced by >4 kg after 52 weeks. Nausea occurred less frequently in this assessment period and was predominantly mild. No major hypoglycemia was observed.
Conclusion: Exenatide QW elicited sustained improvements in glycemic control and body weight through 52 weeks of treatment. Patients switching to exenatide QW experienced further improvements in A1C and FPG, with sustained weight loss.
Trial registration: ClinicalTrials.gov NCT00308139.
Figures

Comment in
-
Comparison of once-weekly with twice-daily exenatide in the treatment of type 2 diabetes (DURATION-1 trial).Expert Opin Pharmacother. 2010 Sep;11(13):2269-71. doi: 10.1517/14656566.2010.497142. Expert Opin Pharmacother. 2010. PMID: 20536293
References
-
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B: Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 - PMC - PubMed
-
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) group. Lancet 1998;352:837–853 - PubMed
-
- Brown JB, Nichols GA, Perry A: The burden of treatment failure in type 2 diabetes. Diabetes Care 2004;27:1535–1540 - PubMed
-
- Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC: UKPDS 26: sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabet Med 1998;15:297–303 - PubMed
-
- Pontiroli AE, Calderara A, Pozza G: Secondary failure of oral hypoglycaemic agents: frequency, possible causes, and management. Diabetes Metab Rev 1994;10:31–43 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

