The tandem inversion duplication in Salmonella enterica: selection drives unstable precursors to final mutation types
- PMID: 20215473
- PMCID: PMC2870977
- DOI: 10.1534/genetics.110.114074
The tandem inversion duplication in Salmonella enterica: selection drives unstable precursors to final mutation types
Abstract
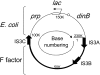
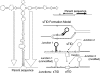
During growth under selection, mutant types appear that are rare in unselected populations. Stress-induced mechanisms may cause these structures or selection may favor a series of standard events that modify common preexisting structures. One such mutation is the short junction (SJ) duplication with long repeats separated by short sequence elements: AB*(CD)*(CD)*E (* = a few bases). Another mutation type, described here, is the tandem inversion duplication (TID), where two copies of a parent sequence flank an inverse-order segment: AB(CD)(E'D'C'B')(CD)E. Both duplication types can amplify by unequal exchanges between direct repeats (CD), and both are rare in unselected cultures but common after prolonged selection for amplification. The observed TID junctions are asymmetric (aTIDs) and may arise from a symmetrical precursor (sTID)-ABCDE(E'D'C'B'A')ABCDE-when sequential deletions remove each palindromic junction. Alternatively, one deletion can remove both sTID junctions to generate an SJ duplication. It is proposed that sTID structures form frequently under all growth conditions, but are usually lost due to their instability and fitness cost. Selection for increased copy number helps retain the sTID and favors deletions that remodel junctions, improve fitness, and allow higher amplification. Growth improves with each step in formation of an SJ or aTID amplification, allowing selection to favor completion of the mutation process.
Figures








Similar articles
-
Multiple pathways of duplication formation with and without recombination (RecA) in Salmonella enterica.Genetics. 2012 Oct;192(2):397-415. doi: 10.1534/genetics.112.142570. Epub 2012 Aug 3. Genetics. 2012. PMID: 22865732 Free PMC article.
-
Multiple pathways of selected gene amplification during adaptive mutation.Proc Natl Acad Sci U S A. 2006 Nov 14;103(46):17319-24. doi: 10.1073/pnas.0608309103. Epub 2006 Nov 2. Proc Natl Acad Sci U S A. 2006. PMID: 17082307 Free PMC article.
-
The effect of genomic position on reversion of a lac frameshift mutation (lacIZ33) during non-lethal selection (adaptive mutation).Mol Microbiol. 2002 May;44(4):1017-32. doi: 10.1046/j.1365-2958.2002.02934.x. Mol Microbiol. 2002. PMID: 12010495
-
Mechanisms of gene duplication and amplification.Cold Spring Harb Perspect Biol. 2015 Feb 2;7(2):a016592. doi: 10.1101/cshperspect.a016592. Cold Spring Harb Perspect Biol. 2015. PMID: 25646380 Free PMC article. Review.
-
Amplification-mutagenesis--how growth under selection contributes to the origin of genetic diversity and explains the phenomenon of adaptive mutation.Res Microbiol. 2004 Jun;155(5):342-51. doi: 10.1016/j.resmic.2004.01.016. Res Microbiol. 2004. PMID: 15207866 Review.
Cited by
-
Chromosomal Replication Complexity: A Novel DNA Metrics and Genome Instability Factor.PLoS Genet. 2016 Oct 6;12(10):e1006229. doi: 10.1371/journal.pgen.1006229. eCollection 2016 Oct. PLoS Genet. 2016. PMID: 27711112 Free PMC article. Review.
-
Multiple pathways of duplication formation with and without recombination (RecA) in Salmonella enterica.Genetics. 2012 Oct;192(2):397-415. doi: 10.1534/genetics.112.142570. Epub 2012 Aug 3. Genetics. 2012. PMID: 22865732 Free PMC article.
-
Genomic plasticity enables phenotypic variation of Pseudomonas syringae pv. tomato DC3000.PLoS One. 2014 Feb 6;9(2):e86628. doi: 10.1371/journal.pone.0086628. eCollection 2014. PLoS One. 2014. PMID: 24516535 Free PMC article.
-
Reinterpreting Long-Term Evolution Experiments: Is Delayed Adaptation an Example of Historical Contingency or a Consequence of Intermittent Selection?J Bacteriol. 2016 Feb 16;198(7):1009-12. doi: 10.1128/JB.00110-16. J Bacteriol. 2016. PMID: 26883821 Free PMC article.
-
Bacteria can compensate the fitness costs of amplified resistance genes via a bypass mechanism.Nat Commun. 2024 Mar 14;15(1):2333. doi: 10.1038/s41467-024-46571-7. Nat Commun. 2024. PMID: 38485998 Free PMC article.
References
-
- Ahmed, A., and L. Podemski, 1998. Observations on template switching during DNA replication through long inverted repeats. Gene 223 187–194. - PubMed
-
- Andersson, D. I., E. S. Slechta and J. R. Roth, 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282 1133–1135. - PubMed
-
- Andersson, D., D. Hughes and J. Roth, 2010. The origin of mutants under selection: interactions of mutation, growth and selection, in EcoSal: Escherichia coli and Salmonella, Cellular and Molecular Biology. ASM Press (in press). - PubMed
-
- Bachellier, S., J. M. Clement and M. Hofnung, 1999. Short palindromic repetitive DNA elements in enterobacteria: a survey. Res. Microbiol. 150 627–639. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

