Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression
- PMID: 20215503
- PMCID: PMC2840052
- DOI: 10.1158/0008-5472.CAN-09-3515
Matrix metalloproteinases contribute distinct roles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesis progression
Abstract
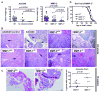
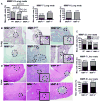
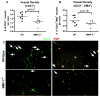
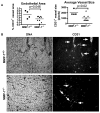
Prostate cancer is the leading form of cancer in men. Prostate tumors often contain neuroendocrine differentiation, which correlates with androgen-independent progression and poor prognosis. Matrix metalloproteinases (MMP), a family of enzymes that remodel the microenvironment, are associated with tumorigenesis and metastasis. To evaluate MMPs during metastatic prostatic neuroendocrine cancer development, we used transgenic mice expressing SV40 large T antigen in their prostatic neuroendocrine cells, under the control of transcriptional regulatory elements from the mouse cryptdin-2 gene (CR2-TAg). These mice have a stereotypical pattern of tumorigenesis and metastasis. MMP-2, MMP-7, and MMP-9 activities increased concurrently with the transition to invasive metastatic carcinoma, but they were expressed in different prostatic cell types: stromal, luminal epithelium, and macrophages, respectively. CR2-TAg mice treated with AG3340/Prinomastat, an MMP inhibitor that blocks activity of MMP-2, MMP-9, MMP-13, and MMP-14, had reduced tumor burden. CR2-TAg animals were crossed to mice homozygous for null alleles of MMP-2, MMP-7, or MMP-9 genes. At 24 weeks CR2-TAg; MMP-2(-/-) mice showed reduced tumor burden, prolonged survival, decreased lung metastasis, and decreased blood vessel density, whereas deficiencies in MMP-7 or MMP-9 did not influence tumor growth or survival. Mice deficient for MMP-7 had reduced endothelial area coverage and decreased vessel size, and mice lacking MMP-9 had increased numbers of invasive foci and increased perivascular invasion, as well as decreased tumor blood vessel size. Together, these results suggest distinct contributions by MMPs to the progression of aggressive prostate tumor and to helping tumors cleverly find alternative routes to malignant progression.
Conflict of interest statement
Figures


Similar articles
-
Expression of matrix metalloproteinase-2 and -9 and their inhibitors, tissue inhibitor of metalloproteinase-1 and -2, in primary cultures of human prostatic stromal and epithelial cells.J Cell Physiol. 2002 May;191(2):208-16. doi: 10.1002/jcp.10092. J Cell Physiol. 2002. PMID: 12064464
-
Quantitative immunohistochemical and in situ hybridization analysis of metalloproteinases in prostate cancer.Anticancer Res. 2006 Mar-Apr;26(2A):973-82. Anticancer Res. 2006. PMID: 16619495
-
Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis.J Natl Cancer Inst. 2002 Jan 2;94(1):17-25. doi: 10.1093/jnci/94.1.17. J Natl Cancer Inst. 2002. PMID: 11773278
-
[Matrix metalloproteinases and colorectal cancer].Med Klin (Munich). 2003 Dec 15;98(12):763-70. doi: 10.1007/s00063-003-1322-5. Med Klin (Munich). 2003. PMID: 14685678 Review. German.
-
Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment.Oncogene. 2000 Dec 27;19(56):6642-50. doi: 10.1038/sj.onc.1204097. Oncogene. 2000. PMID: 11426650 Review.
Cited by
-
Immunoglobulin g from breast cancer patients regulates MCF-7 cells migration and MMP-9 activity by stimulating muscarinic acetylcholine receptors.J Clin Immunol. 2013 Feb;33(2):427-35. doi: 10.1007/s10875-012-9804-y. Epub 2012 Sep 25. J Clin Immunol. 2013. PMID: 23007238
-
Expression and significance of MMP2 and HIF-1α in hepatocellular carcinoma.Oncol Lett. 2014 Aug;8(2):539-546. doi: 10.3892/ol.2014.2189. Epub 2014 May 28. Oncol Lett. 2014. PMID: 25013467 Free PMC article.
-
Regulatory mechanisms of heme regulatory protein BACH1: a potential therapeutic target for cancer.Med Oncol. 2021 Sep 4;38(10):122. doi: 10.1007/s12032-021-01573-z. Med Oncol. 2021. PMID: 34482423 Review.
-
Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization.Int J Cancer. 2011 Jun 1;128(11):2536-44. doi: 10.1002/ijc.26032. Epub 2011 Mar 25. Int J Cancer. 2011. PMID: 21387299 Free PMC article. Review.
-
Increased expression of matrix metalloproteinase-13 in glioma is associated with poor overall survival of patients.Med Oncol. 2012 Dec;29(4):2432-7. doi: 10.1007/s12032-012-0181-4. Epub 2012 Feb 17. Med Oncol. 2012. PMID: 22351249
References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. - PubMed
-
- Turbat-Herrera EA, Herrera GA, Gore I, Lott RL, Grizzle WE, Bonnin JM. Neuroendocrine differentiation in prostatic carcinomas. A retrospective autopsy study. Arch Pathol Lab Med. 1988;112(11):1100–5. - PubMed
-
- Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities. Eur Urol. 2005;47(2):147–55. - PubMed
-
- Komiya A, Suzuki H, Imamoto T, et al. Neuroendocrine differentiation in the progression of prostate cancer. Int J Urol. 2009;16(1):37–44. - PubMed
-
- Bonkhoff H, Stein U, Remberger K. Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic, and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol. 1993;423(4):291–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

