Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin
- PMID: 20215518
- PMCID: PMC2840193
- DOI: 10.1158/0008-5472.CAN-09-3249
Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin
Abstract
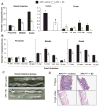
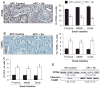
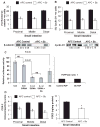
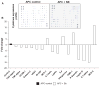
Chemoprevention is a practical and translational approach to reduce the risk of various cancers including colorectal cancer (CRC), which is a major cause of cancer-related deaths in the United States. Accordingly, here we assessed chemopreventive efficacy and associated mechanisms of long-term silibinin feeding on spontaneous intestinal tumorigenesis in the APC(min/+) mice model. Six-week-old APC(min/+) mice were p.o. fed with vehicle control (0.5% carboxymethyl cellulose and 0.025% Tween 20 in distilled water) or 750 mg silibinin/kg body weight in vehicle for 5 d/wk for 13 weeks and then sacrificed. Silibinin feeding strongly prevented intestinal tumorigenesis in terms of polyp formation in proximal, middle, and distal portions of small intestine by 27% (P < 0.001), 34% (P < 0.001), and 49% (P < 0.001), respectively. In colon, we observed 55% (P < 0.01) reduction in number of polyps by silibinin treatment. In size distribution analysis, silibinin showed significant decrease in large-size polyps (>3 mm) by 66% (P < 0.01) and 88% (P < 0.001) in middle and distal portions of small intestine, respectively. More importantly, silibinin caused a complete suppression in >3 mm sized polyps and 92% reduction in >2 to 3 mm sized polyps in colon. Molecular analyses of polyps suggested that silibinin exerts its chemopreventive efficacy by inhibiting cell proliferation, inflammation, and angiogenesis; inducing apoptosis; decreasing beta-catenin levels and transcriptional activity; and modulating the expression profile of cytokines. These results show for the first time the efficacy and associated mechanisms of long-term p.o. silibinin feeding against spontaneous intestinal tumorigenesis in the APC(min/+) mice model, suggesting its chemopreventive potential against intestinal cancers including CRC.
Figures


Similar articles
-
Silibinin suppresses spontaneous tumorigenesis in APC min/+ mouse model by modulating beta-catenin pathway.Pharm Res. 2009 Dec;26(12):2558-67. doi: 10.1007/s11095-009-9968-1. Epub 2009 Sep 25. Pharm Res. 2009. PMID: 19779968 Free PMC article.
-
Inhibition of intestinal adenoma formation in APC(Min/+) mice by Riccardin D, a natural product derived from liverwort plant Dumortiera hirsuta.PLoS One. 2012;7(3):e33243. doi: 10.1371/journal.pone.0033243. Epub 2012 Mar 14. PLoS One. 2012. PMID: 22432006 Free PMC article.
-
Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis.Cancer Res. 2008 Mar 15;68(6):2043-50. doi: 10.1158/0008-5472.CAN-07-6247. Cancer Res. 2008. PMID: 18339887
-
Dietary feeding of grape seed extract prevents intestinal tumorigenesis in APCmin/+ mice.Neoplasia. 2010 Jan;12(1):95-102. doi: 10.1593/neo.91718. Neoplasia. 2010. PMID: 20072658 Free PMC article.
-
Chemoprevention of intestinal adenomatous polyposis by acetyl-11-keto-beta-boswellic acid in APC(Min/+) mice.Int J Cancer. 2013 Jun 1;132(11):2667-81. doi: 10.1002/ijc.27929. Epub 2012 Nov 26. Int J Cancer. 2013. PMID: 23132636
Cited by
-
Ethanol-induced mast cell-mediated inflammation leads to increased susceptibility of intestinal tumorigenesis in the APC Δ468 min mouse model of colon cancer.Alcohol Clin Exp Res. 2013 Jan;37 Suppl 1(0 1):E199-208. doi: 10.1111/j.1530-0277.2012.01894.x. Alcohol Clin Exp Res. 2013. PMID: 23320800 Free PMC article.
-
Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells.Cell Signal. 2012 Dec;24(12):2291-6. doi: 10.1016/j.cellsig.2012.07.009. Epub 2012 Jul 20. Cell Signal. 2012. PMID: 22820499 Free PMC article.
-
Evaluation of Silibinin Effects on the Viability of HepG2 (Human hepatocellular liver carcinoma) and HUVEC (Human Umbilical Vein Endothelial) Cell Lines.Iran J Pharm Res. 2018 Winter;17(1):261-267. Iran J Pharm Res. 2018. PMID: 29755557 Free PMC article.
-
Cancer and Traditional Medicine: An Integrative Approach.Pharmaceuticals (Basel). 2025 Apr 28;18(5):644. doi: 10.3390/ph18050644. Pharmaceuticals (Basel). 2025. PMID: 40430464 Free PMC article. Review.
-
The Dual Role of Dietary Phytochemicals in Oxidative Stress: Implications for Oncogenesis, Cancer Chemoprevention, and ncRNA Regulation.Antioxidants (Basel). 2025 May 22;14(6):620. doi: 10.3390/antiox14060620. Antioxidants (Basel). 2025. PMID: 40563255 Free PMC article. Review.
References
-
- Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–9. - PubMed
-
- Rustgi AK. The genetics of hereditary colon cancer. Genes Dev. 2007;21:2525–38. - PubMed
-
- Hegde MR, Roa BB. Detecting mutations in the APC gene in familial adenomatous polyposis (FAP) Curr Protoc Hum Genet. 2006;Chapter 10(Unit 10):8. - PubMed
-
- Church JM, McGannon E, Hull-Boiner S, et al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum. 1992;35:1170–3. - PubMed
-
- Nugent KP, Spigelman AD, Phillips RK. Life expectancy after colectomy and ileorectal anastomosis for familial adenomatous polyposis. Dis Colon Rectum. 1993;36:1059–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

