Cetuximab-based immunotherapy and radioimmunotherapy of head and neck squamous cell carcinoma
- PMID: 20215534
- PMCID: PMC2848903
- DOI: 10.1158/1078-0432.CCR-09-2495
Cetuximab-based immunotherapy and radioimmunotherapy of head and neck squamous cell carcinoma
Abstract
Purpose: To show the relationship between antibody delivery and therapeutic efficacy in head and neck cancers, in this study we evaluated the pharmacokinetics and pharmacodynamics of epidermal growth factor receptor (EGFR)-targeted immunotherapy and radioimmunotherapy by quantitative positron emission tomography (PET) imaging.
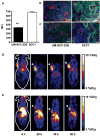
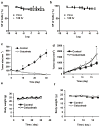
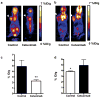
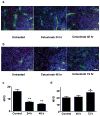
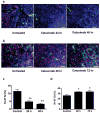
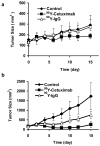
Experimental design: EGFR expression on UM-SCC-22B and SCC1 human head and neck squamous cell cancer (HNSCC) cells were determined by flow cytometry and immunostaining. Tumor delivery and distribution of cetuximab in tumor-bearing nude mice were evaluated with small animal PET using (64)Cu-DOTA-cetuximab. The in vitro toxicity of cetuximab to HNSCC cells was evaluated by MTT assay. The tumor-bearing mice were then treated with four doses of cetuximab at 10 mg/kg per dose, and tumor growth was evaluated by caliper measurement. FDG PET was done after the third dose of antibody administration to evaluate tumor response. Apoptosis and tumor cell proliferation after cetuximab treatment were analyzed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling and Ki-67 staining. Radioimmunotherapy was done with (90)Y-DOTA-cetuximab.
Results: EGFR expression on UM-SCC-22B cells is lower than that on SCC1 cells. However, the UM-SCC-22B tumors showed much higher (64)Cu-DOTA-cetuximab accumulation than the SCC1 tumors. Cetuximab-induced apoptosis in SCC1 tumors and tumor growth was significantly inhibited, whereas an agonistic effect of cetuximab on UM-SCC-22B tumor growth was observed. After cetuximab treatment, the SCC1 tumors showed decreased FDG uptake, and the UM-SCC-22B tumors had increased FDG uptake. UM-SCC-22B tumors are more responsive to (90)Y-DOTA-cetuximab treatment than SCC1 tumors, partially due to the high tumor accumulation of the injected antibody.
Conclusion: Cetuximab has an agonistic effect on the growth of UM-SCC-22B tumors, indicating that tumor response to cetuximab treatment is not necessarily related to EGFR expression and antibody delivery efficiency, as determined by PET imaging. Although PET imaging with antibodies as tracers has limited function in patient screening, it can provide guidance for targeted therapy using antibodies as delivery vehicles.
Copyright 2010 AACR.
Figures
Similar articles
-
Epidermal growth factor receptor-targeted radioimmunotherapy of human head and neck cancer xenografts using 90Y-labeled fully human antibody panitumumab.Mol Cancer Ther. 2010 Aug;9(8):2297-308. doi: 10.1158/1535-7163.MCT-10-0444. Epub 2010 Aug 3. Mol Cancer Ther. 2010. PMID: 20682654 Free PMC article.
-
177Lu-labeled antibodies for EGFR-targeted SPECT/CT imaging and radioimmunotherapy in a preclinical head and neck carcinoma model.Mol Pharm. 2014 Mar 3;11(3):800-7. doi: 10.1021/mp4005047. Epub 2014 Feb 3. Mol Pharm. 2014. PMID: 24472064
-
PET of EGFR antibody distribution in head and neck squamous cell carcinoma models.J Nucl Med. 2009 Jul;50(7):1116-23. doi: 10.2967/jnumed.109.061820. Epub 2009 Jun 12. J Nucl Med. 2009. PMID: 19525473 Free PMC article.
-
Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review.
-
PET Imaging in Head and Neck Cancer Patients to Monitor Treatment Response: A Future Role for EGFR-Targeted Imaging.Clin Cancer Res. 2015 Aug 15;21(16):3602-9. doi: 10.1158/1078-0432.CCR-15-0348. Epub 2015 Apr 30. Clin Cancer Res. 2015. PMID: 25931452 Review.
Cited by
-
Combined treatment of the experimental human papilloma virus-16-positive cervical and head and neck cancers with cisplatin and radioimmunotherapy targeting viral E6 oncoprotein.Br J Cancer. 2013 Mar 5;108(4):859-65. doi: 10.1038/bjc.2013.43. Epub 2013 Feb 5. Br J Cancer. 2013. PMID: 23385729 Free PMC article.
-
Cetuximab augments cytotoxicity with poly (adp-ribose) polymerase inhibition in head and neck cancer.PLoS One. 2011;6(8):e24148. doi: 10.1371/journal.pone.0024148. Epub 2011 Aug 30. PLoS One. 2011. PMID: 21912620 Free PMC article.
-
Mapping Sentinel Lymph Node Metastasis by Dual-probe Optical Imaging.Theranostics. 2017 Jan 1;7(1):153-163. doi: 10.7150/thno.17085. eCollection 2017. Theranostics. 2017. PMID: 28042324 Free PMC article.
-
Longitudinal PET imaging of doxorubicin-induced cell death with 18F-Annexin V.Mol Imaging Biol. 2012 Dec;14(6):762-70. doi: 10.1007/s11307-012-0551-5. Mol Imaging Biol. 2012. PMID: 22392643 Free PMC article.
-
Hypoxia in head and neck cancer in theory and practice: a PET-based imaging approach.Comput Math Methods Med. 2014;2014:624642. doi: 10.1155/2014/624642. Epub 2014 Aug 21. Comput Math Methods Med. 2014. PMID: 25214887 Free PMC article. Review.
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8. - PubMed
-
- Richey LM, Shores CG, George J, et al. The effectiveness of salvage surgery after the failure of primary concomitant chemoradiation in head and neck cancer. Otolaryngol Head Neck Surg. 2007;136:98–103. - PubMed
-
- Argiris A, Stenson KM, Brockstein BE, et al. Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck. 2004;26:447–55. - PubMed
-
- Schoder H, Fury M, Lee N, Kraus D. PET monitoring of therapy response in head and neck squamous cell carcinoma. J Nucl Med. 2009;50(Suppl 1):74S–88S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA067166/CA/NCI NIH HHS/United States
- ZII EB000066/ImNIH/Intramural NIH HHS/United States
- R21 CA102123/CA/NCI NIH HHS/United States
- P01 CA67166/CA/NCI NIH HHS/United States
- R24 CA93862/CA/NCI NIH HHS/United States
- U54 CA119367/CA/NCI NIH HHS/United States
- R01 CA118582/CA/NCI NIH HHS/United States
- PHS CA09302/CA/NCI NIH HHS/United States
- P50CA114747/CA/NCI NIH HHS/United States
- T32 CA009302/CA/NCI NIH HHS/United States
- Z99 EB999999/ImNIH/Intramural NIH HHS/United States
- P50 CA114747/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

