Therapeutic potential of Janus-activated kinase-2 inhibitors for the management of myelofibrosis
- PMID: 20215535
- PMCID: PMC5017533
- DOI: 10.1158/1078-0432.CCR-09-2836
Therapeutic potential of Janus-activated kinase-2 inhibitors for the management of myelofibrosis
Abstract
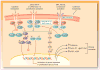
Myelofibrosis (either primary or postpolycythemia vera/essential thrombocythemia) is a chronic and debilitating myeloproliferative neoplasm for which there is no well-accepted standard of care. Clinical manifestations of this disease (e.g., cytopenias, splenomegaly, bone marrow fibrosis) and constitutional symptoms (e.g., hypercatabolic state, fatigue, night sweats, fever) create significant treatment challenges. For example, progressive splenomegaly increases the risk for more serious clinical sequelae (e.g., portal hypertension, splenic infarction). Myelofibrosis arises from hematopoietic stem cells or early progenitor cells. However, the molecular mechanisms underlying its pathogenesis and clinical presentation are poorly understood, delaying the development of effective and targeted treatments. Recent studies have implicated mutations that directly or indirectly lead to the deregulated activation of Janus-activated kinase 2 (JAK2). Appreciation for the activation of JAK2 and the importance of increased levels of circulating proinflammatory cytokines in the pathogenesis and clinical manifestations of myelofibrosis has led to novel therapeutic agents targeting JAKs. This review will briefly discuss the origins of the JAK2 hypothesis, the clinical relevance of JAK2 mutations in myelofibrosis, and recent clinical progress in targeting JAKs as a therapeutic intervention for patients with this chronic and debilitating disease.
Copyright 2010 AACR.
Figures

Similar articles
-
Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis.N Engl J Med. 2010 Sep 16;363(12):1117-27. doi: 10.1056/NEJMoa1002028. N Engl J Med. 2010. PMID: 20843246 Free PMC article. Clinical Trial.
-
Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study.Lancet Haematol. 2017 Jul;4(7):e317-e324. doi: 10.1016/S2352-3026(17)30088-1. Epub 2017 Jun 8. Lancet Haematol. 2017. PMID: 28602585 Free PMC article. Clinical Trial.
-
A phase 1 study of the Janus kinase 2 (JAK2)V617F inhibitor, gandotinib (LY2784544), in patients with primary myelofibrosis, polycythemia vera, and essential thrombocythemia.Leuk Res. 2017 Oct;61:89-95. doi: 10.1016/j.leukres.2017.08.010. Epub 2017 Aug 31. Leuk Res. 2017. PMID: 28934680 Free PMC article. Clinical Trial.
-
The diagnosis and management of polycythemia vera, essential thrombocythemia, and primary myelofibrosis in the JAK2 V617F era.Clin Adv Hematol Oncol. 2009 May;7(5):334-42. Clin Adv Hematol Oncol. 2009. PMID: 19521323 Review.
-
Splenomegaly in myelofibrosis--new options for therapy and the therapeutic potential of Janus kinase 2 inhibitors.J Hematol Oncol. 2012 Aug 1;5:43. doi: 10.1186/1756-8722-5-43. J Hematol Oncol. 2012. PMID: 22852872 Free PMC article. Review.
Cited by
-
Screening for hotspot mutations in PI3K, JAK2, FLT3 and NPM1 in patients with myelodysplastic syndromes.Clinics (Sao Paulo). 2011;66(5):793-9. doi: 10.1590/s1807-59322011000500014. Clinics (Sao Paulo). 2011. PMID: 21789382 Free PMC article.
-
The Network of Inflammatory Mechanisms in Lupus Nephritis.Front Med (Lausanne). 2020 Nov 6;7:591724. doi: 10.3389/fmed.2020.591724. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33240910 Free PMC article. Review.
-
Targeting Janus Kinases and Signal Transducer and Activator of Transcription 3 to Treat Inflammation, Fibrosis, and Cancer: Rationale, Progress, and Caution.Pharmacol Rev. 2020 Apr;72(2):486-526. doi: 10.1124/pr.119.018440. Pharmacol Rev. 2020. PMID: 32198236 Free PMC article. Review.
-
Practical management of patients with myelofibrosis receiving ruxolitinib.Expert Rev Hematol. 2013 Oct;6(5):511-23. doi: 10.1586/17474086.2013.827413. Epub 2013 Oct 2. Expert Rev Hematol. 2013. PMID: 24083419 Free PMC article. Review.
-
A Case of Chronic Myelogenous Leukemia Occurring in a Patient Treated for Essential Thrombocythemia.Am J Case Rep. 2019 Jan 3;20:10-14. doi: 10.12659/AJCR.911854. Am J Case Rep. 2019. PMID: 30602717 Free PMC article.
References
-
- Mesa RA. Navigating the evolving paradigms in the diagnosis and treatment of myeloproliferative disorders. Hematology Am Soc Hematol Educ Program. 2007;2007:355–62. - PubMed
-
- Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006;355:2452–66. - PubMed
-
- Spivak JL, Barosi G, Tognoni G, et al. Chronic myeloproliferative disorders. Hematology Am Soc Hematol Educ Program. 2003:200–24. - PubMed
-
- Tefferi A, Huang J, Schwager S, et al. Validation and comparison of contemporary prognostic models in primary myelofibrosis: analysis based on 334 patients from a single institution. Cancer. 2007;109:2083–8. - PubMed
-
- Wolanskyj AP, Schwager SM, McClure RF, Larson DR, Tefferi A. Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc. 2006;81:159–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

