Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial
- PMID: 20215554
- PMCID: PMC2840067
- DOI: 10.1158/1078-0432.CCR-09-2980
Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial
Abstract
Purpose: Although most children with B-lineage acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma are cured, new agents are needed to overcome drug resistance and reduce toxicities of chemotherapy. We hypothesized that the novel anti-CD22 immunotoxin, RFB4(dsFv)-PE38 (BL22, CAT-3888), would be active and have limited nonspecific side effects in children with CD22-expressing hematologic malignancies. We conducted the first preclinical and phase I clinical studies of BL22 in that setting.
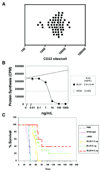
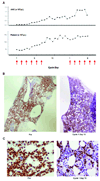
Experimental design: Lymphoblasts from children with B-lineage ALL were assessed for CD22 expression by flow cytometry and for BL22 sensitivity by in vitro cytotoxicity assay. BL22 was evaluated in a human ALL murine xenograft model. A phase I clinical trial was conducted for pediatric subjects with CD22+ ALL and non-Hodgkin lymphoma.
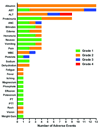
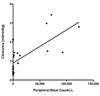
Results: All samples screened were CD22+. BL22 was cytotoxic to blasts in vitro (median IC(50), 9.8 ng/mL) and prolonged the leukemia-free survival of murine xenografts. Phase I trial cohorts were treated at escalating doses and schedules ranging from 10 to 40 microg/kg every other day for three or six doses repeated every 21 or 28 days. Treatment was associated with an acceptable safety profile, adverse events were rapidly reversible, and no maximum tolerated dose was defined. Pharmacokinetics were influenced by disease burden consistent with rapid drug binding by CD22+ blasts. Although no responses were observed, transient clinical activity was seen in most subjects.
Conclusions: CD22 represents an excellent target and anti-CD22 immunotoxins offer therapeutic promise in B-lineage hematologic malignancies of childhood.
Conflict of interest statement
Robert J. Kreitman, David J. FitzGerald, and Ira Pastan are co-inventors on patents assigned to the NIH for the investigational product used in this research.
Figures
References
-
- Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst. 2008;100:1301–1309. - PubMed
-
- Smith MA, Gloeckler Ries LA, Gurney JG, Ross JA. Reis LAG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program, 1999. Bethesda: MD: 1999. Leukemia. pp. 17–34. NIH Pub No 99-4649.
-
- Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005;131:579–587. - PubMed
-
- Gloeckler Ries LA. Reis LAG, Smith MA, Gurney JG, et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995, National Cancer Institute, SEER Program, 1999. Bethesda: MD: 1999. Childhood cancer mortality. pp. 165–170. NIH Pub No 99-4649.
-
- Pui C-H, Cheng C, Leung W, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

