Glucose intolerance and impaired insulin secretion in pancreas-specific signal transducer and activator of transcription-3 knockout mice are associated with microvascular alterations in the pancreas
- PMID: 20215569
- PMCID: PMC2869255
- DOI: 10.1210/en.2009-1199
Glucose intolerance and impaired insulin secretion in pancreas-specific signal transducer and activator of transcription-3 knockout mice are associated with microvascular alterations in the pancreas
Abstract
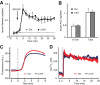
Maintenance of glucose homeostasis depends on adequate amount and precise pattern of insulin secretion, which is determined by both beta-cell secretory processes and well-developed microvascular network within endocrine pancreas. The development of highly organized microvasculature and high degrees of capillary fenestrations in endocrine pancreas is greatly dependent on vascular endothelial growth factor-A (VEGF-A) from islet cells. However, it is unclear how VEGF-A production is regulated in endocrine pancreas. To understand whether signal transducer and activator of transcription (STAT)-3 is involved in VEGF-A regulation and subsequent islet and microvascular network development, we generated a mouse line carrying pancreas-specific deletion of STAT3 (p-KO) and performed physiological analyses both in vivo and using isolated islets, including glucose and insulin tolerance tests, and insulin secretion measurements. We also studied microvascular network and islet development by using immunohistochemical methods. The p-KO mice exhibited glucose intolerance and impaired insulin secretion in vivo but normal insulin secretion in isolated islets. Microvascular density in the pancreas was reduced in p-KO mice, along with decreased expression of VEGF-A, but not other vasotropic factors in islets in the absence of pancreatic STAT3 signaling. Together, our study suggests that pancreatic STAT3 signaling is required for the normal development and maintenance of endocrine pancreas and islet microvascular network, possibly through its regulation of VEGF-A.
Figures

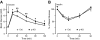


Similar articles
-
Altered islet morphology but normal islet secretory function in vitro in a mouse model with microvascular alterations in the pancreas.PLoS One. 2013 Jul 29;8(7):e71277. doi: 10.1371/journal.pone.0071277. Print 2013. PLoS One. 2013. PMID: 23923060 Free PMC article.
-
Pancreatic islet production of vascular endothelial growth factor--a is essential for islet vascularization, revascularization, and function.Diabetes. 2006 Nov;55(11):2974-85. doi: 10.2337/db06-0690. Diabetes. 2006. PMID: 17065333
-
Pancreatic T cell protein-tyrosine phosphatase deficiency affects beta cell function in mice.Diabetologia. 2015 Jan;58(1):122-31. doi: 10.1007/s00125-014-3413-7. Epub 2014 Oct 23. Diabetologia. 2015. PMID: 25338551 Free PMC article.
-
Microvascular development: learning from pancreatic islets.Bioessays. 2004 Oct;26(10):1069-75. doi: 10.1002/bies.20105. Bioessays. 2004. PMID: 15382139 Review.
-
Role of VEGF-A in pancreatic beta cells.Endocr J. 2010;57(3):185-91. doi: 10.1507/endocrj.k09e-035. Epub 2010 Feb 24. Endocr J. 2010. PMID: 20179357 Review.
Cited by
-
High Glucose Induces Down-Regulated GRIM-19 Expression to Activate STAT3 Signaling and Promote Cell Proliferation in Cell Culture.PLoS One. 2016 Apr 21;11(4):e0153659. doi: 10.1371/journal.pone.0153659. eCollection 2016. PLoS One. 2016. PMID: 27101310 Free PMC article.
-
Mechanisms and regulation of endothelial VEGF receptor signalling.Nat Rev Mol Cell Biol. 2016 Oct;17(10):611-25. doi: 10.1038/nrm.2016.87. Epub 2016 Jul 27. Nat Rev Mol Cell Biol. 2016. PMID: 27461391 Review.
-
STAT3 modulates β-cell cycling in injured mouse pancreas and protects against DNA damage.Cell Death Dis. 2016 Jun 23;7(6):e2272. doi: 10.1038/cddis.2016.171. Cell Death Dis. 2016. PMID: 27336716 Free PMC article.
-
Identification of Novel Regulatory Regions Induced by Intrauterine Growth Restriction in Rat Islets.Endocrinology. 2022 Feb 1;163(2):bqab251. doi: 10.1210/endocr/bqab251. Endocrinology. 2022. PMID: 34894232 Free PMC article.
-
Talin-1 inhibits Smurf1-mediated Stat3 degradation to modulate β-cell proliferation and mass in mice.Cell Death Dis. 2023 Oct 31;14(10):709. doi: 10.1038/s41419-023-06235-8. Cell Death Dis. 2023. PMID: 37903776 Free PMC article.
References
-
- Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P 1994 Stimulus-secretion coupling in pancreatic β cells. J Cell Biochem 55(Suppl):54–65 - PubMed
-
- Rorsman P, Renström E 2003 Insulin granule dynamics in pancreatic β cells. Diabetologia 46:1029–1045 - PubMed
-
- Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC 2002 Signals and pools underlying biphasic insulin secretion. Diabetes 51(Suppl 1):S60–S67 - PubMed
-
- Carlsson PO, Andersson A, Jansson L 1996 Pancreatic islet blood flow in normal and obese-hyperglycemic (ob/ob) mice. Am J Physiol 271:E990–E995 - PubMed
-
- Svensson AM, Sandler S, Jansson L 2003 Role of superoxide anion in pancreatic islet blood flow regulation in anesthetized rats. Eur J Pharmacol 459:59–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

