A novel role for ABCA1-generated large pre-beta migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion
- PMID: 20215580
- PMCID: PMC2842152
- DOI: 10.1194/jlr.M900083
A novel role for ABCA1-generated large pre-beta migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion
Abstract
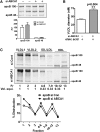
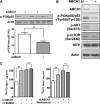
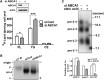
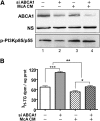
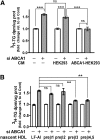
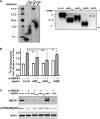
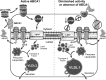
In Tangier disease, absence of ATP binding cassette transporter A1 (ABCA1) results in reduced plasma HDL and elevated triglyceride (TG) levels. We hypothesized that hepatocyte ABCA1 regulates VLDL TG secretion through nascent HDL production. Silencing of ABCA1 expression in oleate-stimulated rat hepatoma cells resulted in: 1) decreased large nascent HDL (>10 nm diameter) and increased small nascent HDL (<10 nm) formation, 2) increased large buoyant VLDL1 particle secretion, and 3) decreased phosphatidylinositol-3 (PI3) kinase activation. Nascent HDL-containing conditioned medium from rat hepatoma cells or HEK293 cells transfected with ABCA1 was effective in increasing PI3 kinase activation and reducing VLDL TG secretion in ABCA1-silenced hepatoma cells. Addition of isolated large nascent HDL particles to ABCA1-silenced hepatoma cells inhibited VLDL TG secretion to a greater extent than small nascent HDL. Similarly, addition of recombinant HDL, but not human plasma HDL, was effective in attenuating TG secretion and increasing PI3 kinase activation in ABCA1-silenced cells. Collectively, these data suggest that large nascent HDL particles, assembled by hepatic ABCA1, generate a PI3 kinase-mediated autocrine signal that attenuates VLDL maturation and TG secretion. This pathway may explain the elevated plasma TG concentration that occurs in most Tangier subjects and may also account, in part, for the inverse relationship between plasma HDL and TG concentrations in individuals with compromised ABCA1 function.
Figures

Similar articles
-
Hepatic ABCA1 deficiency is associated with delayed apolipoprotein B secretory trafficking and augmented VLDL triglyceride secretion.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt A):1035-1043. doi: 10.1016/j.bbalip.2017.07.001. Epub 2017 Jul 8. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28694219 Free PMC article.
-
Hepatic ABCA1 and VLDL triglyceride production.Biochim Biophys Acta. 2012 May;1821(5):770-7. doi: 10.1016/j.bbalip.2011.09.020. Epub 2011 Oct 6. Biochim Biophys Acta. 2012. PMID: 22001232 Free PMC article. Review.
-
Targeted deletion of hepatocyte ABCA1 leads to very low density lipoprotein triglyceride overproduction and low density lipoprotein hypercatabolism.J Biol Chem. 2010 Apr 16;285(16):12197-209. doi: 10.1074/jbc.M109.096933. Epub 2010 Feb 23. J Biol Chem. 2010. PMID: 20178985 Free PMC article.
-
ATP-Binding Cassette Transporter A1 Deficiency in Human Induced Pluripotent Stem Cell-Derived Hepatocytes Abrogates HDL Biogenesis and Enhances Triglyceride Secretion.EBioMedicine. 2017 Apr;18:139-145. doi: 10.1016/j.ebiom.2017.03.018. Epub 2017 Mar 14. EBioMedicine. 2017. PMID: 28330813 Free PMC article.
-
Hepatic ABC transporters and triglyceride metabolism.Curr Opin Lipidol. 2012 Jun;23(3):196-200. doi: 10.1097/MOL.0b013e328352dd1a. Curr Opin Lipidol. 2012. PMID: 22488425 Free PMC article. Review.
Cited by
-
Effect of adiponectin on macrophage reverse cholesterol transport in adiponectin-/- mice and its mechanism.Exp Ther Med. 2017 Jun;13(6):2757-2762. doi: 10.3892/etm.2017.4321. Epub 2017 Apr 10. Exp Ther Med. 2017. PMID: 28587337 Free PMC article.
-
APOE and KLF14 genetic variants are sex-specific for low high-density lipoprotein cholesterol identified by a genome-wide association study.Genet Mol Biol. 2022 Feb 21;45(1):e20210280. doi: 10.1590/1678-4685-GMB-2021-0280. eCollection 2022. Genet Mol Biol. 2022. PMID: 35238325 Free PMC article.
-
Role of ABCA1 in Cardiovascular Disease.J Pers Med. 2022 Jun 20;12(6):1010. doi: 10.3390/jpm12061010. J Pers Med. 2022. PMID: 35743794 Free PMC article. Review.
-
Association of ATP-Binding Cassette Transporter A1 (ABCA1)-565 C/T Gene Polymorphism with Hypoalphalipoproteinemia and Serum Lipids, IL-6 and CRP Levels.Avicenna J Med Biotechnol. 2017 Jan-Mar;9(1):38-43. Avicenna J Med Biotechnol. 2017. PMID: 28090279 Free PMC article.
-
Hepatic ABCA1 deficiency is associated with delayed apolipoprotein B secretory trafficking and augmented VLDL triglyceride secretion.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt A):1035-1043. doi: 10.1016/j.bbalip.2017.07.001. Epub 2017 Jul 8. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28694219 Free PMC article.
References
-
- Broccardo C., Luciani M., Chimini G. 1999. The ABCA subclass of mammalian transporters. Biochim. Biophys. Acta. 1461: 395–404. - PubMed
-
- Assman G., von Eckardstein A., Brewer H. B., Jr 2001. Familial Analphalipoproteinemia: Tangier Disease. The Metabolic and Molecular Bases of Inherited Disease. Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K. W., Volkman B. F., McGraw-Hill, New York: 2937–2960.
-
- Brooks-Wilson A., Marcil M., Clee S.M., Zhang L.H., Roomp K., van Dam M., Yu C., Brewer J. A., Collins H. O., Molhuizen O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345. - PubMed
-
- Rust S., Rosier M., Funke H., Real J., Amoura Z., Piette J. C., Deleuze J. F., Brewer H. B., Duverger N., Denefle P., et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22: 352–355. - PubMed
-
- Bodzioch M., Orso E., Klucken J., Langmann T., Bottcher A., Diederich W., Drobnik W., Barlage S., Buchler C., Porsch-Ozcurumez M., et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22: 347–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

