Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization
- PMID: 20215588
- PMCID: PMC2861451
- DOI: 10.1105/tpc.109.071746
Arabidopsis homologs of nucleus- and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization
Abstract
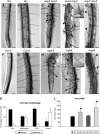
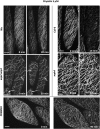
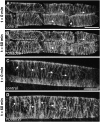
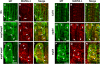
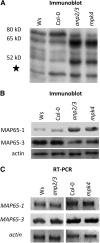
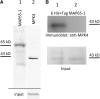
A double homozygous recessive mutant in the Arabidopsis thaliana homologs of nucleus- and phragmoplast-localized kinase 2 (ANP2) and 3 (ANP3) genes and a homozygous recessive mutant in the mitogen-activated protein kinase 4 (MPK4) gene of Arabidopsis exhibit deficiencies in the overall microtubule (MT) organization, which result in abnormal cell growth patterns, such as branching of root hairs and swelling of diffusely growing epidermal cells. Genetic, pharmacological, molecular, cytological, and biochemical analyses show that the major underlying mechanism for these phenotypes is excessive MT stabilization manifested in both mutants as heavy MT bundling, disorientation, and drug stability. The above defects in MAPK signaling result in the adverse regulation of members of the microtubule-associated protein (MAP65) protein family, including strongly diminished phosphorylation of MAP65-1. These data suggest that ANP2/ANP3, MPK4, and the microtubule-associated protein MAP65-1, a putative target of MPK4 signaling, are all essential for the proper organization of cortical microtubules in Arabidopsis epidermal cells.
Figures





References
-
- Akhmanova A., Steinmetz M.O. (2008). Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9: 309–322 - PubMed
-
- Bao Y., Kost B., Chua N.H. (2001). Reduced expression of alpha-tubulin genes in Arabidopsis thaliana specifically affects root growth and morphology, root hair development and root gravitropism. Plant J. 28: 145–157 - PubMed
-
- Baskin T.I., Wilson J.E., Cork A., Williamson R.E. (1994). Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol. 35: 935–942 - PubMed
-
- Bibikova T.N., Blancaflor E.B., Gilroy S. (1999). Microtubules regulate tip growth and orientation in root hairs of Arabidopsis thaliana. Plant J. 17: 657–665 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

