Contributions of nucleotide excision repair, DNA polymerase eta, and homologous recombination to replication of UV-irradiated herpes simplex virus type 1
- PMID: 20215648
- PMCID: PMC2859539
- DOI: 10.1074/jbc.M110.107920
Contributions of nucleotide excision repair, DNA polymerase eta, and homologous recombination to replication of UV-irradiated herpes simplex virus type 1
Abstract
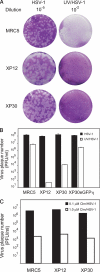
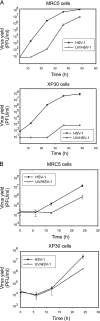
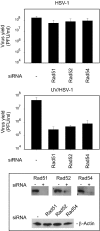
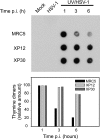
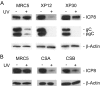
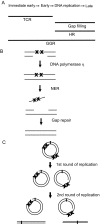
The effects of UV irradiation on herpes simplex virus type 1 (HSV-1) gene expression and DNA replication were examined in cell lines containing mutations inactivating the XPA gene product required for nucleotide-excision repair, the DNA polymerase eta responsible for translesion synthesis, or the Cockayne syndrome A and B (CSA and CSB) gene products required for transcription-coupled nucleotide excision repair. In the absence of XPA and CSA and CSB gene products, virus replication was reduced 10(6)-, 400-, and 100-fold, respectively. In DNA polymerase eta mutant cells HSV-1 plaque efficiency was reduced 10(4)-fold. Furthermore, DNA polymerase eta was strictly required for virus replication at low multiplicities of infection but dispensable at high multiplicities of infection. Knock down of Rad 51, Rad 52, and Rad 54 levels by RNA interference reduced replication of UV-irradiated HSV-1 150-, 100-, and 50-fold, respectively. We find that transcription-coupled repair efficiently supports expression of immediate early and early genes from UV-irradiated HSV-1 DNA. In contrast, the progression of the replication fork appears to be impaired, causing a severe reduction of late gene expression. Since the HSV-1 replisome does not make use of proliferating cell nuclear antigen, we attribute the replication defect to an inability to perform proliferating cell nuclear antigen-dependent translesion synthesis by polymerase switching at the fork. Instead, DNA polymerase eta may act during postreplication gap filling. Homologous recombination, finally, might restore the physical and genetic integrity of the virus chromosome.
Figures
Similar articles
-
A postincision-deficient TFIIH causes replication fork breakage and uncovers alternative Rad51- or Pol32-mediated restart mechanisms.Mol Cell. 2010 Mar 12;37(5):690-701. doi: 10.1016/j.molcel.2010.02.008. Mol Cell. 2010. PMID: 20227372
-
Opposing effects of the UV lesion repair protein XPA and UV bypass polymerase eta on ATR checkpoint signaling.EMBO J. 2006 Jun 7;25(11):2605-14. doi: 10.1038/sj.emboj.7601123. Epub 2006 May 4. EMBO J. 2006. PMID: 16675950 Free PMC article.
-
Transcription-coupled nucleotide excision repair is coordinated by ubiquitin and SUMO in response to ultraviolet irradiation.Nucleic Acids Res. 2020 Jan 10;48(1):231-248. doi: 10.1093/nar/gkz977. Nucleic Acids Res. 2020. PMID: 31722399 Free PMC article.
-
HSV-1 DNA Replication-Coordinated Regulation by Viral and Cellular Factors.Viruses. 2021 Oct 7;13(10):2015. doi: 10.3390/v13102015. Viruses. 2021. PMID: 34696446 Free PMC article. Review.
-
Common pathways for ultraviolet skin carcinogenesis in the repair and replication defective groups of xeroderma pigmentosum.J Dermatol Sci. 2000 May;23(1):1-11. doi: 10.1016/s0923-1811(99)00088-2. J Dermatol Sci. 2000. PMID: 10699759 Review.
Cited by
-
Early nucleosome deposition on, and replication of, HSV DNA requires cell factor PCNA.J Neurovirol. 2015 Aug;21(4):358-69. doi: 10.1007/s13365-015-0321-7. Epub 2015 Feb 12. J Neurovirol. 2015. PMID: 25672886 Free PMC article.
-
Herpes Simplex Virus 1 Manipulates Host Cell Antiviral and Proviral DNA Damage Responses.mBio. 2021 Feb 9;12(1):e03552-20. doi: 10.1128/mBio.03552-20. mBio. 2021. PMID: 33563816 Free PMC article.
-
Recombination promoted by DNA viruses: phage λ to herpes simplex virus.Annu Rev Microbiol. 2014;68:237-58. doi: 10.1146/annurev-micro-091313-103424. Epub 2014 Jun 9. Annu Rev Microbiol. 2014. PMID: 25002096 Free PMC article. Review.
-
Rad51 and Rad52 are involved in homologous recombination of replicating herpes simplex virus DNA.PLoS One. 2014 Nov 3;9(11):e111584. doi: 10.1371/journal.pone.0111584. eCollection 2014. PLoS One. 2014. PMID: 25365323 Free PMC article.
-
Identification of conserved amino acids in the herpes simplex virus type 1 UL8 protein required for DNA synthesis and UL52 primase interaction in the virus replisome.J Biol Chem. 2012 Sep 28;287(40):33142-52. doi: 10.1074/jbc.M112.356782. Epub 2012 Jul 30. J Biol Chem. 2012. PMID: 22851167 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

