The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells
- PMID: 20215869
- PMCID: PMC3678275
- DOI: 10.4161/chan.4.3.11223
The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells
Abstract
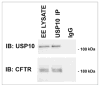
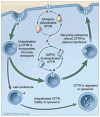
The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) is a cyclic AMP-regulated chloride channel that plays an important role in regulating the volume of the lung airway surface liquid, and thereby mucociliary clearance and elimination of pathogens from the lung. In epithelial cells, cell surface CFTR abundance is determined in part by regulating both CFTR endocytosis from the apical plasma membrane and recycling back to the plasma membrane. We recently reported, using an activity-based chemical screen to identify active deubiquitinating enzymes (DUBs) in human airway epithelial cells, that Ubiquitin Specific Protease-10 (USP10) is located and active in the early endosomal compartment and regulates the deubiquitination of CFTR and thereby promotes its endocytic recycling. siRNA-mediated knockdown of USP10 increased the multi-ubiquitination and lysosomal degradation of CFTR and decreased the endocytic recycling and the half-life of CFTR in the apical membrane, as well as CFTR-mediated chloride secretion. Overexpression of wild-type USP10 reduced CFTR multi-ubiquitination and degradation, while overexpression of a dominant-negative USP10 promoted increased multi-ubiquitination and lysosomal degradation of CFTR. In the current study, we show localization and activity of USP10 in the early endosomal compartment of primary bronchial epithelial cells, as well as an interaction between CFTR and USP10 in this compartment. These studies demonstrate a novel function for USP10 in facilitating the deubiquitination of CFTR in early endosomes, thereby enhancing the endocytic recycling and cell surface expression of CFTR.
Figures

Similar articles
-
The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells.J Biol Chem. 2009 Jul 10;284(28):18778-89. doi: 10.1074/jbc.M109.001685. Epub 2009 Apr 27. J Biol Chem. 2009. PMID: 19398555 Free PMC article.
-
Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval.PLoS One. 2014 Feb 21;9(2):e89599. doi: 10.1371/journal.pone.0089599. eCollection 2014. PLoS One. 2014. PMID: 24586903 Free PMC article.
-
c-Cbl facilitates endocytosis and lysosomal degradation of cystic fibrosis transmembrane conductance regulator in human airway epithelial cells.J Biol Chem. 2010 Aug 27;285(35):27008-27018. doi: 10.1074/jbc.M110.139881. Epub 2010 Jun 4. J Biol Chem. 2010. PMID: 20525683 Free PMC article.
-
The role of regulated CFTR trafficking in epithelial secretion.Am J Physiol Cell Physiol. 2003 Jul;285(1):C1-18. doi: 10.1152/ajpcell.00554.2002. Am J Physiol Cell Physiol. 2003. PMID: 12777252 Review.
-
Regulated trafficking of the CFTR chloride channel.Eur J Cell Biol. 2000 Aug;79(8):544-56. doi: 10.1078/0171-9335-00078. Eur J Cell Biol. 2000. PMID: 11001491 Review.
Cited by
-
Trafficking and function of the cystic fibrosis transmembrane conductance regulator: a complex network of posttranslational modifications.Am J Physiol Lung Cell Mol Physiol. 2016 Oct 1;311(4):L719-L733. doi: 10.1152/ajplung.00431.2015. Epub 2016 Jul 29. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27474090 Free PMC article. Review.
-
Modulation of Retrograde Trafficking of KCa3.1 in a Polarized Epithelium.Front Physiol. 2017 Jul 18;8:489. doi: 10.3389/fphys.2017.00489. eCollection 2017. Front Physiol. 2017. PMID: 28769813 Free PMC article.
-
USP10 as a Potential Therapeutic Target in Human Cancers.Genes (Basel). 2022 May 6;13(5):831. doi: 10.3390/genes13050831. Genes (Basel). 2022. PMID: 35627217 Free PMC article. Review.
-
Endocytosis: A Turnover Mechanism Controlling Ion Channel Function.Cells. 2020 Aug 4;9(8):1833. doi: 10.3390/cells9081833. Cells. 2020. PMID: 32759790 Free PMC article. Review.
-
A targeted in vivo RNAi screen reveals deubiquitinases as new regulators of Notch signaling.G3 (Bethesda). 2012 Dec;2(12):1563-75. doi: 10.1534/g3.112.003780. Epub 2012 Dec 1. G3 (Bethesda). 2012. PMID: 23275879 Free PMC article.
References
-
- Swiatecka-Urban A, Brown A, Moreau-Marquis S, et al. The short apical membrane half-life of rescued ΔF508-cystic fibrosis transmembrane conductance regulator (CFTR) results from accelerated endocytosis of ΔF508-CFTR in polarized human airway epithelial cells. J Biol Chem. 2005;280:23–5. - PubMed
-
- Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J. 2004;23:146–58. - PubMed
-
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–72. - PubMed
-
- Clague MJ, Urbe S. Endocytosis: the DUB version. Trends Cell Biol. 2006;16:551–9. - PubMed
-
- Mukhopadhyay D, Riezman H. Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science. 2007;315:201–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
