Serum markers may distinguish biliary atresia from other forms of neonatal cholestasis
- PMID: 20216099
- PMCID: PMC2881691
- DOI: 10.1097/MPG.0b013e3181cb42ee
Serum markers may distinguish biliary atresia from other forms of neonatal cholestasis
Abstract
Background: Biliary atresia (BA) is the most serious liver disease in infants. Diagnosis currently depends on surgical exploration of the biliary tree. Noninvasive tests that distinguish BA from other types of neonatal liver disease are not available.
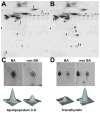
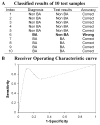
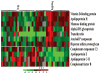
Patients and methods: To identify potential serum biomarkers that classify children with neonatal cholestasis, we performed 2-dimensional difference gel electrophoresis, statistical analysis, and tandem mass spectrometry using serum samples from 19 infants with BA and 19 infants with non-BA neonatal cholestasis.
Results: Eleven potential serum biomarkers were found that could in combination classify children with neonatal cholestasis.
Conclusions: Although no single biomarker or imaging test adequately distinguishes BA from other types of neonatal cholestasis, combinations of biomarkers, imaging tests, and noninvasive clinical criteria should be further explored as potential tests for rapid and accurate diagnosis of BA.
Figures

Similar articles
-
Circulating microRNA is a biomarker of biliary atresia.J Pediatr Gastroenterol Nutr. 2012 Oct;55(4):366-9. doi: 10.1097/MPG.0b013e318264e648. J Pediatr Gastroenterol Nutr. 2012. PMID: 22732895 Free PMC article.
-
Identification of HSP90 as potential biomarker of biliary atresia using two-dimensional electrophoresis and mass spectrometry.PLoS One. 2013 Jul 11;8(7):e68602. doi: 10.1371/journal.pone.0068602. Print 2013. PLoS One. 2013. PMID: 23874684 Free PMC article.
-
Liquid chromatography-mass spectroscopy in the diagnosis of biliary atresia in children with hyperbilirubinemia.J Surg Res. 2018 Aug;228:228-237. doi: 10.1016/j.jss.2018.03.021. Epub 2018 Apr 11. J Surg Res. 2018. PMID: 29907216
-
Role of Hepatobiliary Scintigraphy and Preoperative Liver Biopsy for Exclusion of Biliary Atresia in Neonatal Cholestasis Syndrome.Indian J Pediatr. 2017 Sep;84(9):685-690. doi: 10.1007/s12098-017-2408-z. Epub 2017 Jul 8. Indian J Pediatr. 2017. PMID: 28687948 Review.
-
Biliary Atresia/Neonatal Cholestasis: What is in the Horizon?Pediatr Clin North Am. 2021 Dec;68(6):1333-1341. doi: 10.1016/j.pcl.2021.08.002. Pediatr Clin North Am. 2021. PMID: 34736593 Review.
Cited by
-
Diagnostic value of procalcitonin and apo-e in extrahepatic biliary atresia.Iran J Pediatr. 2014 Oct;24(5):623-9. Epub 2014 Feb 18. Iran J Pediatr. 2014. PMID: 25793072 Free PMC article.
-
Circulating microRNA is a biomarker of biliary atresia.J Pediatr Gastroenterol Nutr. 2012 Oct;55(4):366-9. doi: 10.1097/MPG.0b013e318264e648. J Pediatr Gastroenterol Nutr. 2012. PMID: 22732895 Free PMC article.
-
Development and Validation of Novel Diagnostic Models for Biliary Atresia in a Large Cohort of Chinese Patients.EBioMedicine. 2018 Aug;34:223-230. doi: 10.1016/j.ebiom.2018.07.025. Epub 2018 Aug 1. EBioMedicine. 2018. PMID: 30077722 Free PMC article.
-
Digital camera image analysis of faeces in detection of cholestatic jaundice in infants.Afr J Paediatr Surg. 2016 Jul-Sep;13(3):131-5. doi: 10.4103/0189-6725.187810. Afr J Paediatr Surg. 2016. PMID: 27502881 Free PMC article.
-
Serum microRNA microarray analysis identifies miR-4429 and miR-4689 are potential diagnostic biomarkers for biliary atresia.Sci Rep. 2016 Feb 16;6:21084. doi: 10.1038/srep21084. Sci Rep. 2016. PMID: 26879603 Free PMC article.
References
-
- de Carvalho E, Ivantes CA, Bezerra JA. Extrahepatic biliary atresia: current concepts and future directions. J Pediatr (Rio J) 2007;83(2):105–120. - PubMed
-
- Sokol RJ, Mack C, Narkewicz MR, Karrer FM. Pathogenesis and outcome of biliary atresia: current concepts. J Pediatr Gastroenterol Nutr. 2003;37(1):4–21. - PubMed
-
- Wadhwani SI, Turmelle YP, Nagy R, Lowell J, Dillon P, Shepherd RW. Prolonged neonatal jaundice and the diagnosis of biliary atresia: a single-center analysis of trends in age at diagnosis and outcomes. Pediatrics. 2008;121(5):e1438–1440. - PubMed

