Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa
- PMID: 20216425
- PMCID: PMC2880466
- DOI: 10.1097/QAI.0b013e3181cf9979
Effects of highly active antiretroviral therapy duration and regimen on risk for mother-to-child transmission of HIV in Johannesburg, South Africa
Abstract
Background: Limited information exists about effects of different highly active antiretroviral therapy (HAART) regimens and duration of regimens on mother-to-child transmission (MTCT) of HIV among women in Africa who start treatment for advanced immunosuppression.
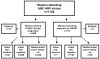
Methods: Between January 2004 to August 2008, 1142 women were followed at antenatal antiretroviral clinics in Johannesburg. Predictors of MTCT (positive infant HIV DNA polymerase chain reaction at 4-6 weeks) were assessed with multivariate logistic regression.
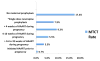
Results: Mean age was 30.2 years (SD = 5.0) and median baseline CD4 count was 161 cells per cubic millimeter (SD = 84.3). HAART duration at time of delivery was a mean 10.7 weeks (SD = 7.4) for the 85% of women who initiated treatment during pregnancy and 93.4 weeks (SD = 37.7) for those who became pregnant on HAART. Overall MTCT rate was 4.9% (43 of 874), with no differences detected between HAART regimens. MTCT rates were lower in women who became pregnant on HAART than those initiating HAART during pregnancy (0.7% versus 5.7%; P = 0.01). In the latter group, each additional week of treatment reduced odds of transmission by 8% (95% confidence interval: 0.87 to 0.99, P = 0.02).
Conclusions: Late initiation of HAART is associated with increased risk of MTCT. Strategies are needed to facilitate earlier identification of HIV-infected women.
Figures
Similar articles
-
Optimal time on HAART for prevention of mother-to-child transmission of HIV.J Acquir Immune Defic Syndr. 2011 Oct 1;58(2):224-8. doi: 10.1097/QAI.0b013e318229147e. J Acquir Immune Defic Syndr. 2011. PMID: 21709566 Free PMC article.
-
Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d'Ivoire.AIDS. 2008 Sep 12;22(14):1815-20. doi: 10.1097/QAD.0b013e32830b8ab9. AIDS. 2008. PMID: 18753864
-
Risk factors for detectable HIV-1 RNA at delivery among women receiving highly active antiretroviral therapy in the women and infants transmission study.J Acquir Immune Defic Syndr. 2010 May 1;54(1):27-34. doi: 10.1097/QAI.0b013e3181caea89. J Acquir Immune Defic Syndr. 2010. PMID: 20065861 Free PMC article.
-
Current treatment strategies, complications and considerations for the use of HIV antiretroviral therapy during pregnancy.AIDS Rev. 2011 Oct-Dec;13(4):198-213. AIDS Rev. 2011. PMID: 21975356 Review.
-
Safety of agents used to prevent mother-to-child transmission of HIV: is there any cause for concern?Drug Saf. 2007;30(3):203-13. doi: 10.2165/00002018-200730030-00004. Drug Saf. 2007. PMID: 17343429 Review.
Cited by
-
Predictive factors of plasma HIV suppression during pregnancy: a prospective cohort study in Benin.PLoS One. 2013;8(3):e59446. doi: 10.1371/journal.pone.0059446. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23555035 Free PMC article. Clinical Trial.
-
Growing inequities in maternal health in South Africa: a comparison of serial national household surveys.BMC Pregnancy Childbirth. 2016 Sep 1;16(1):256. doi: 10.1186/s12884-016-1048-z. BMC Pregnancy Childbirth. 2016. PMID: 27581489 Free PMC article.
-
A tool for estimating antiretroviral medication coverage for HIV-infected women during pregnancy (PMTCT-ACT).Glob Health Res Policy. 2019 Oct 15;4:29. doi: 10.1186/s41256-019-0121-3. eCollection 2019. Glob Health Res Policy. 2019. PMID: 31637308 Free PMC article.
-
Health system weaknesses constrain access to PMTCT and maternal HIV services in South Africa: a qualitative enquiry.AIDS Res Ther. 2011 Mar 3;8:10. doi: 10.1186/1742-6405-8-10. AIDS Res Ther. 2011. PMID: 21371301 Free PMC article.
-
Peripartum HIV infection in very low birth weight infants fed 'raw' mother's own milk.South Afr J HIV Med. 2019 Jun 19;20(1):912. doi: 10.4102/sajhivmed.v20i1.912. eCollection 2019. South Afr J HIV Med. 2019. PMID: 31308967 Free PMC article.
References
-
- Wiktor SZ, Ekpini E, Karon JM, et al. Short-course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomised trial. Lancet. 1999 Mar 6;353(9155):781–5. - PubMed
-
- Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004 Jul 15;351(3):217–28. - PubMed
-
- Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: results from 13 perinatal studies. The Working Group on Mother-To-Child Transmission of HIV. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Apr 15;8(5):506–10. - PubMed
-
- Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. Aids. 2007 Jul;21 4:S65–71. - PubMed
-
- Perinatal HIV Guidelines Working Group. Public Health Service Task Force. 2009. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States; pp. 1–90. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

