Particle tracking of intracellular trafficking of octaarginine-modified liposomes: a comparative study with adenovirus
- PMID: 20216528
- PMCID: PMC2890115
- DOI: 10.1038/mt.2010.33
Particle tracking of intracellular trafficking of octaarginine-modified liposomes: a comparative study with adenovirus
Abstract
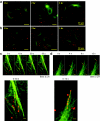
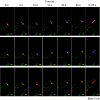
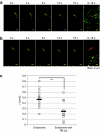
It is previously reported that octaarginine (R8)-modified liposome (R8-Lip) was taken up via macropinocytosis, and subsequently delivered to the nuclear periphery. In the present study, we investigated the mechanism for the cytoplasmic transport of R8-Lips, comparing with that for adenovirus. Treatment with microtubule-disruption reagent (nocodazole) inhibited the transfection activity of plasmid DNA (pDNA)-encapsulating R8-Lip more extensively than that of adenovirus. The directional transport of R8-Lips along green fluorescent protein (GFP)-tagged microtubules was observed; however, the velocity was slower than those for adenovirus or endosomes that were devoid of R8-Lips. These directional motions were abrogated in R8-Lips by nocodazole treatment, whereas adenovirus continued to undergo random motion. This finding suggests that the nuclear access of R8-Lip predominantly involves microtubule-dependent transport, whereas an apparent diffusive motion is also operative in nuclear access of adenovirus. Furthermore, quantum dot-labeled pDNA underwent directional motion concomitantly with rhodamine-labeled lipid envelopes, indicating that the R8-Lips were subject to microtubule-dependent transport in the intact form. Dual particle tracking of carriers and endosomes revealed that R8-Lip was directionally transported, associated with endosomes, whereas this occurs after endosomal escape in adenovirus. Collectively, the findings reported herein indicate that vesicular transport is a key factor in the cytoplasmic transport of R8-Lips.
Figures




Similar articles
-
Intracellular fate of octaarginine-modified liposomes in polarized MDCK cells.Int J Pharm. 2010 Feb 15;386(1-2):122-30. doi: 10.1016/j.ijpharm.2009.11.005. Epub 2009 Nov 13. Int J Pharm. 2010. PMID: 19922779
-
Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes.Mol Ther. 2008 Aug;16(8):1507-14. doi: 10.1038/mt.2008.122. Epub 2008 Jun 17. Mol Ther. 2008. PMID: 18560420
-
High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression.J Biol Chem. 2006 Feb 10;281(6):3544-51. doi: 10.1074/jbc.M503202200. Epub 2005 Dec 2. J Biol Chem. 2006. PMID: 16326716
-
[Development of a novel artificial gene delivery system multifunctional envelope-type nano device for gene therapy].Yakugaku Zasshi. 2007 Oct;127(10):1685-91. doi: 10.1248/yakushi.127.1685. Yakugaku Zasshi. 2007. PMID: 17917425 Review. Japanese.
-
Octaarginine-modified liposomes: enhanced cellular uptake and controlled intracellular trafficking.Int J Pharm. 2008 Apr 16;354(1-2):39-48. doi: 10.1016/j.ijpharm.2007.12.003. Epub 2007 Dec 14. Int J Pharm. 2008. PMID: 18242018 Review.
Cited by
-
Quantitative nanoparticle tracking: applications to nanomedicine.Nanomedicine (Lond). 2011 Jun;6(4):693-700. doi: 10.2217/nnm.11.42. Nanomedicine (Lond). 2011. PMID: 21718178 Free PMC article. Review.
-
Development of an image Mean Square Displacement (iMSD)-based method as a novel approach to study the intracellular trafficking of nanoparticles.Acta Biomater. 2016 Sep 15;42:189-198. doi: 10.1016/j.actbio.2016.07.031. Epub 2016 Jul 20. Acta Biomater. 2016. PMID: 27449340 Free PMC article.
-
Cytoplasmic trafficking, endosomal escape, and perinuclear accumulation of adeno-associated virus type 2 particles are facilitated by microtubule network.J Virol. 2012 Oct;86(19):10462-73. doi: 10.1128/JVI.00935-12. Epub 2012 Jul 18. J Virol. 2012. PMID: 22811523 Free PMC article.
-
Synthetic Approaches for Nucleic Acid Delivery: Choosing the Right Carriers.Life (Basel). 2019 Jul 9;9(3):59. doi: 10.3390/life9030059. Life (Basel). 2019. PMID: 31324016 Free PMC article. Review.
-
Gene Electrotransfer: A Mechanistic Perspective.Curr Gene Ther. 2016;16(2):98-129. doi: 10.2174/1566523216666160331130040. Curr Gene Ther. 2016. PMID: 27029943 Free PMC article. Review.
References
-
- Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90:604–610. - PubMed
-
- Vivès E, Brodin P., and , Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. - PubMed
-
- Wadia JS, Stan RV., and , Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. - PubMed
-
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. - PubMed
-
- Suzuki T, Futaki S, Niwa M, Tanaka S, Ueda K., and , Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J Biol Chem. 2002;277:2437–2443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

