Oxaliplatin, irinotecan and capecitabine as first-line therapy in metastatic colorectal cancer (mCRC): a dose-finding study and pharmacogenomic analysis
- PMID: 20216541
- PMCID: PMC2844042
- DOI: 10.1038/sj.bjc.6605595
Oxaliplatin, irinotecan and capecitabine as first-line therapy in metastatic colorectal cancer (mCRC): a dose-finding study and pharmacogenomic analysis
Abstract
Background: A dose-finding study was performed to evaluate the dose-limiting toxicity (DLT), maximum-tolerated dose (MTD) and the recommended dose (RD) of escalating the doses of capecitabine and fixed doses of irinotecan and oxaliplatin on a biweekly schedule for metastatic colorectal cancer patients (mCRC). A pharmacogenomic analysis was performed to investigate the association between SNPs and treatment outcome.
Methods: Eighty-seven chemotherapy-naïve mCRC patients were recruited through a two-step study design; 27 were included in the dose-finding study and 60 in the pharmacogenomic analysis. Oxaliplatin (85 mg m(-2)) and CPT-11 (150 mg m(-2)), both on day 1, and capecitabine doses ranging from 850 to 1500 mg m(-2) bid on days 1-7 were explored. Peripheral blood samples were used to genotype 13 SNPs in 10 genes related to drug metabolism or efficacy. Univariate and multivariate Cox analysis was performed to examine associations between SNPs, ORR and PFS.
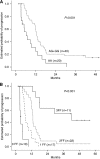
Results: The capecitabine RD was 1000 mg m(-2) bid. Diarrhoea and neutropenia were the DLTs. After a median follow-up of 52.5 months, the median PFS and OS were 12 (95% CI; 10.6-13.4) and 27 months (95% CI; 17.2-36.8), respectively.The GSTP1-G genotype, the Köhne low-risk category and use of a consolidation approach strongly correlated with decreased risk of progression. Patients with all favourable variables showed a median PFS of 42 months vs 3.4 months in the group with all adverse factors. A superior clinical response was obtained in patients with one GSTP1-G allele as compared with GSTP1-AA carriers (P=0.004).
Conclusion: First-line therapy with oxaliplatin, irinotecan and capecitabine is efficient and well-tolerated. The GSTP1 polymorphism A>G status was significantly associated with ORR and PFS in mCRC treated with this triplet therapy.
Figures
Similar articles
-
Feasibility of biweekly combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in patients with metastatic solid tumors: results of a two-step phase I trial: XELIRI and XELIRINOX.Cancer Chemother Pharmacol. 2012 Mar;69(3):807-14. doi: 10.1007/s00280-011-1764-z. Epub 2011 Oct 30. Cancer Chemother Pharmacol. 2012. PMID: 22037922 Clinical Trial.
-
A dose-escalation study of oxaliplatin/capecitabine/irinotecan (XELOXIRI) and bevacizumab as a first-line therapy for patients with metastatic colorectal cancer.Cancer Chemother Pharmacol. 2015 Mar;75(3):587-94. doi: 10.1007/s00280-014-2672-9. Epub 2015 Jan 11. Cancer Chemother Pharmacol. 2015. PMID: 25577134 Clinical Trial.
-
A phase Ib dose-escalation study of erlotinib, capecitabine and oxaliplatin in metastatic colorectal cancer patients.Ann Oncol. 2008 Feb;19(2):332-9. doi: 10.1093/annonc/mdm452. Epub 2007 Nov 6. Ann Oncol. 2008. PMID: 17986625 Clinical Trial.
-
Capecitabine plus oxaliplatin for the treatment of colorectal cancer.Expert Rev Anticancer Ther. 2008 Feb;8(2):161-74. doi: 10.1586/14737140.8.2.161. Expert Rev Anticancer Ther. 2008. PMID: 18279056 Review.
-
Current status of capecitabine in the treatment of colorectal cancer.Oncology (Williston Park). 2002 Dec;16(12 Suppl No 14):16-22. Oncology (Williston Park). 2002. PMID: 12520635 Review.
Cited by
-
Platinum-induced neurotoxicity and preventive strategies: past, present, and future.Oncologist. 2015 Apr;20(4):411-32. doi: 10.1634/theoncologist.2014-0044. Epub 2015 Mar 12. Oncologist. 2015. PMID: 25765877 Free PMC article. Review.
-
Revisiting Platinum-Based Anticancer Drugs to Overcome Gliomas.Int J Mol Sci. 2021 May 12;22(10):5111. doi: 10.3390/ijms22105111. Int J Mol Sci. 2021. PMID: 34065991 Free PMC article. Review.
-
How to select the optimal treatment for first line metastatic colorectal cancer.World J Gastroenterol. 2014 Jan 28;20(4):899-907. doi: 10.3748/wjg.v20.i4.899. World J Gastroenterol. 2014. PMID: 24574764 Free PMC article. Review.
-
DEK is a potential marker for aggressive phenotype and irinotecan-based therapy response in metastatic colorectal cancer.BMC Cancer. 2014 Dec 16;14:965. doi: 10.1186/1471-2407-14-965. BMC Cancer. 2014. PMID: 25515240 Free PMC article.
-
Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis.J Clin Oncol. 2014 Apr 1;32(10):1031-9. doi: 10.1200/JCO.2013.51.1857. Epub 2014 Mar 3. J Clin Oncol. 2014. PMID: 24590654 Free PMC article.
References
-
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O, Levi F, Bismuth H (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 240: 644–657 - PMC - PubMed
-
- Bajetta E, Celio L, Ferrario E, Di Bartolomeo M, Denaro A, Dotti K, Mancin M, Bajetta R, Colombo A, Pusceddu S (2007) Capecitabine plus oxaliplatin and irinotecan regimen every other week: a phase I/II study in first-line treatment of metastatic colorectal cancer. Ann Oncol 18: 1810–1816 - PubMed
-
- Braun MS, Richman SD, Thompson L, Daly CL, Meade AM, Adlard JW, Allan JM, Parmar MK, Quirke P, Seymour MT (2009) Association of molecular markers with toxicity outcomes in a randomized trial of chemotherapy for advanced colorectal cancer: the FOCUS trial. J Clin Oncol 27(33): 5519–5528 - PubMed
-
- Bray F, Sankila R, Ferlay J, Parkin DM (2002) Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 38: 99–166 - PubMed
-
- Calvo E, Cortés J, González-Cao M, Rodríguez J, Aramendía JM, Fernández-Hidalgo O, Martín-Algarra S, Salgado JE, Martínez-Monge R, de Irala J, Brugarolas A (2002a) Combined irinotecan, oxaliplatin and 5-fluorouracil in patients with advanced colorectal cancer. A feasibility pilot study. Oncology 63: 254–265 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous