Acid-sensing ion channels in acidosis-induced injury of human brain neurons
- PMID: 20216553
- PMCID: PMC2916164
- DOI: 10.1038/jcbfm.2010.30
Acid-sensing ion channels in acidosis-induced injury of human brain neurons
Abstract
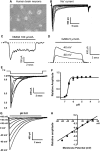
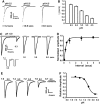
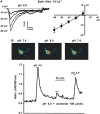
Acidosis is a common feature of the human brain during ischemic stroke and is known to cause neuronal injury. However, the mechanism underlying acidosis-mediated injury of the human brain remains elusive. We show that a decrease in the extracellular pH evoked inward currents characteristic of acid-sensing ion channels (ASICs) and increased intracellular Ca(2+) in cultured human cortical neurons. Acid-sensing ion channels in human cortical neurons show electrophysiological and pharmacological properties distinct from those in neurons of the rodent brain. Reverse transcriptase-PCR and western blot detected a high level of the ASIC1a subunit with little or no expression of other ASIC subunits. Treatment of human cortical neurons with acidic solution induced substantial cell injury, which was attenuated by the ASIC1a blockade. Thus, functional homomeric ASIC1a channels are predominantly expressed in neurons from the human brain. Activation of these channels has an important role in acidosis-mediated injury of human brain neurons.
Figures




References
-
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. ASIC2 modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296–18305. - PubMed
-
- Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton- gated channels sensitive to Gd3+ J Biol Chem. 2000;275:28519–28525. - PubMed
-
- Babinski K, Le KT, Seguela P. Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J Neurochem. 1999;72:51–57. - PubMed
-
- Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

