Identification of a radical formed in the reaction mixtures of ram seminal vesicle microsomes with arachidonic Acid using high performance liquid chromatography-electron spin resonance spectrometry and high performance liquid chromatography-electron spin resonance-mass spectrometry
- PMID: 20216946
- PMCID: PMC2831092
- DOI: 10.3164/jcbn.09-90
Identification of a radical formed in the reaction mixtures of ram seminal vesicle microsomes with arachidonic Acid using high performance liquid chromatography-electron spin resonance spectrometry and high performance liquid chromatography-electron spin resonance-mass spectrometry
Abstract
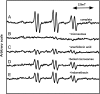
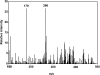
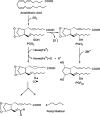
The reaction of ram seminal vesicle (RSV) microsomes with arachidonic acid (AA) was examined using electron spin resonance (ESR), high performance liquid chromatography-electron spin resonance spectrometry (HPLC-ESR), and high performance liquid chromatography-electron spin resonance-mass spectrometry (HPLC-ESR-MS) combined use of spin trapping technique. A prominent ESR spectrum (alpha(N) = 1.58 mT and alpha(H)beta = 0.26 mT) was observed in the complete reaction mixture of ram seminal vesicle microsomes with arachidonic acid containing 2.0 mg protein/ml ram seminal vesicle (RSV) microsomal suspension, 0.8 mM arachidonic acid, 0.1 M 4-POBN, and 24 mM tris/HCl buffer (pH 7.4). The ESR spectrum was hardly observed for the complete reaction mixture without the RSV microsomes. The formation of the radical appears to be catalyzed by the microsomal components. In the absence of AA, the intensity of the ESR signal decreased to 16 +/- 15% of the complete reaction mixture, suggesting that the radical is derived from AA. For the complete reaction mixture with boiled microsomes, the intensity of the ESR signal decreased to 49 +/- 4% of the complete reaction mixture. The intensity of the ESR signal of the complete reaction mixture with indomethacin decreased to 74 +/- 20% of the complete reaction mixture, suggesting that cyclooxygenese partly participates in the reaction. A peak was detected on the elution profile of HPLC-ESR analysis of the complete reaction mixture. To determine the structure of the peak, an HPLC-ESR-MS analysis was performed. The HPLC-ESR-MS analysis of the peak showed two prominent ions, m/z 266 and m/z 179, suggesting that the peak is a 4-POBN/pentyl radical adduct. An HPLC-ESR analysis of the authentic 4-POBN/pentyl radical adduct comfirmed the identification.
Keywords: COX; ESR; arachidonic acid; peroxidation; radical.
Figures

Similar articles
-
Identification of radicals formed in the reaction mixtures of rat liver microsomes with ADP, Fe3+ and NADPH using HPLC EPR and HPLC EPR MS.J Biochem. 2007 Jul;142(1):73-8. doi: 10.1093/jb/mvm114. Epub 2007 Jul 23. J Biochem. 2007. PMID: 17646184
-
Identification of a radical formed in the reaction mixtures of oxidized phosphatidylcholine with ferrous ions using HPLC-ESR and HPLC-ESR-MS.Free Radic Res. 2005 Sep;39(9):987-93. doi: 10.1080/10715760500232073. Free Radic Res. 2005. PMID: 16087480
-
Isolation and identification of alpha-(4-pyridyl-1-oxide)-N-tert-butylnitrone radical adducts formed by the decomposition of the hydroperoxides of linoleic acid, linolenic acid, and arachidonic acid by soybean lipoxygenase.Arch Biochem Biophys. 1991 Feb 15;285(1):172-80. doi: 10.1016/0003-9861(91)90346-k. Arch Biochem Biophys. 1991. PMID: 1846731
-
An advanced Electron Spin Resonance (ESR) spin-trapping and LC/(ESR)/MS technique for the study of lipid peroxidation.Int J Mol Sci. 2012 Nov 12;13(11):14648-66. doi: 10.3390/ijms131114648. Int J Mol Sci. 2012. PMID: 23203086 Free PMC article. Review.
-
Standardization of glycan elution on high-performance liquid chromatography.2022 Jan 18 [updated 2022 Mar 24]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2022 Jan 18 [updated 2022 Mar 24]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590781 Free Books & Documents. Review. No abstract available.
References
-
- Smith W.L., Murphy R.C. In: The eicosanoids: cyclooxygenase, lipoxygenase, and epoxygenase pathways, in Biochemistry of Lipids, Lipoproteins and Membranes, 5th edition. Vance D.E., Vance J.E., editors. Elsevier; Amsterdam: 2008. pp. 331–362.
-
- Smith W.L., DeWitt D.L., Garavito R.M. Cyclooxygenase: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. - PubMed
-
- Kam P.C., See A.U. Cyclo-oxygenase isoenzymes: physiological and pharmacological role. Anaesthesia. 2000;55:442–449. - PubMed
-
- Katori M., Majima M. Cyclooxygenase-2: its rich diversity of roles and possible application of its selective inhibitors. Inflamm. Res. 2000;49:367–392. - PubMed
-
- Rouzer C.A., Marnett L.J. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem. Rev. 2003;103:2239–2304. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

