Extensive Analysis of Elastase-Induced Pulmonary Emphysema in Rats: ALP in the Lung, a New Biomarker for Disease Progression?
- PMID: 20216950
- PMCID: PMC2831096
- DOI: 10.3164/jcbn.09-87
Extensive Analysis of Elastase-Induced Pulmonary Emphysema in Rats: ALP in the Lung, a New Biomarker for Disease Progression?
Abstract
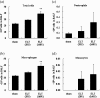
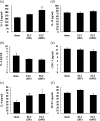
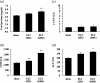
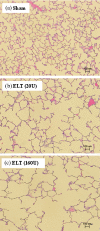
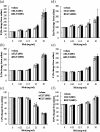
It is accepted that pulmonary exposure of rodents to porcine pancreatic elastase (ELT) induces lesions that morphologically resemble human emphysema. Nonetheless, extensive analysis of this model has rarely been conducted. The present study was designed to extensively examine the effects of ELT on lung inflammation, cell damage, emphysematous change, and cholinergic reactivity in rats. Intratracheal administration of two doses of ELT induced 1) a proinflammatory response in the lung that was characterized by significant infiltration of macrophages and an increased level of interleukin-1beta in lung homogenates, 2) lung cell damage as indicated by higher levels of total protein, lactate dehydrogenase, and alkaline phosphatase (ALP) in lung homogenates, 3) emphysema-related morphological changes including airspace enlargement and progressive destruction of alveolar wall structures, and 4) airway responsiveness to methacholine including an augmented Rn value. In addition, ELT at a high dose was more effective than that at a low dose. This is the novel study to extensively analyze ELT-induced lung emphysema, and the analysis might be applied to future investigations that evaluate new therapeutic agents or risk factors for pulmonary emphysema. In particular, ALP in lung homogenates might be a new biomarker for the disease progression/exacerbation.
Keywords: ALP; LDH; airway hyperresponsiveness; pulmonary emphysema; rat.
Figures
Similar articles
-
Therapeutic effects of LASSBio-596 in an elastase-induced mouse model of emphysema.Front Physiol. 2015 Sep 30;6:267. doi: 10.3389/fphys.2015.00267. eCollection 2015. Front Physiol. 2015. PMID: 26483698 Free PMC article.
-
Comprehensive analysis of elastase-induced pulmonary emphysema in mice: effects of ambient existing particulate matters.Int Immunopharmacol. 2010 Nov;10(11):1380-9. doi: 10.1016/j.intimp.2010.07.016. Epub 2010 Aug 26. Int Immunopharmacol. 2010. PMID: 20800710
-
Emphysema requires the receptor for advanced glycation end-products triggering on structural cells.Am J Respir Cell Mol Biol. 2015 Apr;52(4):482-91. doi: 10.1165/rcmb.2014-0027OC. Am J Respir Cell Mol Biol. 2015. PMID: 25188021
-
Putative role of neutrophil elastase in the pathogenesis of emphysema.Ann N Y Acad Sci. 1991;624:45-59. doi: 10.1111/j.1749-6632.1991.tb17005.x. Ann N Y Acad Sci. 1991. PMID: 2064248 Review.
-
The pulmonary matrix, glycosaminoglycans and pulmonary emphysema.Connect Tissue Res. 1999;40(2):97-104. doi: 10.3109/03008209909029105. Connect Tissue Res. 1999. PMID: 10761634 Review.
Cited by
-
Compensatory lung growth after bilobectomy in emphysematous rats.PLoS One. 2017 Jul 27;12(7):e0181819. doi: 10.1371/journal.pone.0181819. eCollection 2017. PLoS One. 2017. PMID: 28750097 Free PMC article.
-
Elastase-coupled beads as a tool for characterizing localized alveolar tissue destruction associated with the onset of emphysema.J Appl Physiol (1985). 2013 Jun;114(11):1637-44. doi: 10.1152/japplphysiol.00026.2013. Epub 2013 Apr 4. J Appl Physiol (1985). 2013. PMID: 23558388 Free PMC article.
-
Reduction of Emphysema Severity by Human Umbilical Cord-Derived Mesenchymal Stem Cells in Mice.Int J Mol Sci. 2022 Aug 10;23(16):8906. doi: 10.3390/ijms23168906. Int J Mol Sci. 2022. PMID: 36012176 Free PMC article.
-
Fibrotic response of tissue remodeling in COPD.Lung. 2011 Apr;189(2):101-9. doi: 10.1007/s00408-011-9279-2. Epub 2011 Feb 2. Lung. 2011. PMID: 21287182 Review.
-
Therapeutic effects of LASSBio-596 in an elastase-induced mouse model of emphysema.Front Physiol. 2015 Sep 30;6:267. doi: 10.3389/fphys.2015.00267. eCollection 2015. Front Physiol. 2015. PMID: 26483698 Free PMC article.
References
-
- Croxton T.L., Weinmann G.G., Senior R.M., Hoidal J.R. Future research directions in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;165:838–844. - PubMed
-
- Mannino D.M., Buist A.S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. - PubMed
-
- Birrell M.A., Wong S., Hele D.J., McCluskie K., Hardaker E., Belvisi M.G. Steroid-resistant inflammation in a rat model of chronic obstructive pulmonary disease is associated with a lack of nuclear factor-kappaB pathway activation. Am. J. Respir. Crit. Care Med. 2005;172:74–84. - PubMed
-
- Mandl I., Darnule T.V., Fierer J.A., Keller S., Turino G.M. Elastin degradation in human and experimental emphysema. Adv. Exp. Med. Biol. 1977;79:221–231. - PubMed

