Entering and exiting the Medicare part D coverage gap: role of comorbidities and demographics
- PMID: 20217267
- PMCID: PMC2869422
- DOI: 10.1007/s11606-010-1300-6
Entering and exiting the Medicare part D coverage gap: role of comorbidities and demographics
Abstract
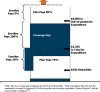
Background: Some Medicare Part D enrollees whose drug expenditures exceed a threshold enter a coverage gap with full cost-sharing, increasing their risk for reduced adherence and adverse outcomes.
Objective: To examine comorbidities and demographic characteristics associated with gap entry and exit.
Design: We linked 2005-2006 pharmacy, outpatient, and inpatient claims to enrollment and Census data. We used logistic regression to estimate associations of 2006 gap entry and exit with 2005 medical comorbidities, demographics, and Census block characteristics. We expressed all results as predicted percentages.
Patients: 287,713 patients without gap coverage, continuously enrolled in a Medicare Advantage Part D (MAPD) plan serving eight states. Patients who received a low-income subsidy, could not be geocoded, or had no 2006 drug fills were excluded.
Results: Of enrollees, 15.9% entered the gap, 2.6% within the first 180 days; among gap enterers, only 6.7% exited again. Gap entry was significantly associated with female gender and all comorbidities, particularly dementia (39.5% gap entry rate) and diabetes (28.0%). Among dementia patients entering the gap, anti-dementia drugs (donepezil, memantine, rivastigmine, and galantamine) and atypical antipsychotic medications (risperidone, quetiapine, and olanzapine) together accounted for 40% of pre-gap expenditures. Among diabetic patients, rosiglitazone accounted for 7.2% of pre-gap expenditures. Having dementia was associated with twice the risk of gap exit.
Conclusions: Certain chronically ill MAPD enrollees are at high risk of gap entry and exposure to unsubsidized medication costs. Clinically vulnerable populations should be counseled on how to best manage costs through drug substitution or discontinuation of specific, non-essential medications.
Conflict of interest statement
None disclosed.
Figures
References
-
- Federal Register. Medicare Program Part D Final Rule, Medicare drug benefit effective CY 2006 (Title 1). Fed Regist [Rules and Regulations], Jan 28, 2005. 70:4193–4585. Available at: http://edocket.access.gpo.gov/2005/pdf/05-1321.pdf. Accessed January 15, 2010.
-
- Hoadley J, Hargrave E, Merrell K, Cubanski J, Neuman T. Benefit design and formularies of Medicare Drug Plans: a comparison of 2006 and 2007 offerings (Menlo Park, Calif.: Henry J. Kaiser Family Foundation, Nov. 2006). Available at: http://www.kff.org/Medicare/upload/7589.pdf. Accessed: January 15, 2010.
-
- Summer L, Nemore P, Finberg J. Improving the Medicare Part D Program for the most vulnerable beneficiaries. 2007 May. Available at: http://www.commonwealthfund.org/publications/publications_show.htm?doc_i.... Accessed January 15, 2010.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous