C5a promotes migration, proliferation, and vessel formation in endothelial cells
- PMID: 20217457
- PMCID: PMC2902742
- DOI: 10.1007/s00011-010-0178-4
C5a promotes migration, proliferation, and vessel formation in endothelial cells
Abstract
Objectives: The goal of this paper is to investigate the effects of activated complement C5a on vascular endothelium during vessel formation.
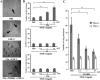
Methods: A human microvascular endothelial cell line (HMEC-1) derived from post-capillary venules in skin was used to measure DNA synthesis, proliferation and cell-cycle progression. In vitro ring-shaped formation by the cells was assessed by using type I collagen gel matrix and a cell-migration assay using the Chemotaxicell chamber. A Matrigel plug assay was performed to confirm the effect of C5a in vivo.
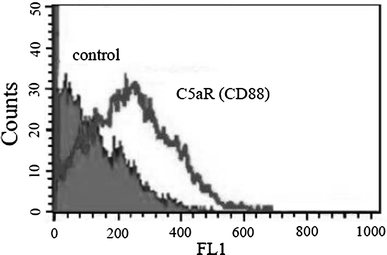
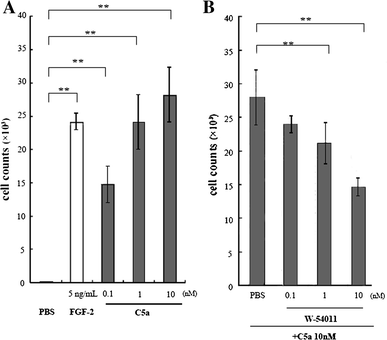
Results: C5a progressed the cell cycle of HMEC-1 into G2/M phases, and induced DNA synthesis and proliferation in a dose-dependent manner. C5a efficiently induced migration and ring-shaped structure formation both in vitro and in vivo. Furthermore, a C5a receptor antagonist (W-54011) suppressed all HMEC-1 activities including proliferation and migration.
Conclusions: Proliferation, migration, and ring-shaped formation by HMEC-1 cells was induced by C5a. The actions were efficiently inhibited by a specific antagonist against C5a. Our results implicated C5a in vessel formation and as a potent target for management of inflammatory diseases.
Figures
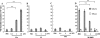
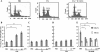
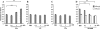

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

