Characterization of tamulamides A and B, polyethers isolated from the marine dinoflagellate Karenia brevis
- PMID: 20218657
- PMCID: PMC2881462
- DOI: 10.1021/np900541w
Characterization of tamulamides A and B, polyethers isolated from the marine dinoflagellate Karenia brevis
Abstract
Florida red tides occur as the result of blooms of the marine dinoflagellate Karenia brevis. K. brevis is known to produce several families of fused polyether ladder compounds. The most notable compounds are the brevetoxins, potent neurotoxins that activate mammalian sodium channels. Additional fused polyether ladder compounds produced by K. brevis include brevenal, brevisin, and hemibrevetoxin B, all with varying affinities for the same binding site on voltage-sensitive sodium channels. The structure elucidation and biological activity of two additional fused polyether ladder compounds containing seven fused ether rings will be described in this paper. Tamulamide A (MW = 638.30) and tamulamide B (MW = 624.29) were isolated from K. brevis cultures, and their structures elucidated using a combination of NMR spectroscopy and high-resolution mass spectrometry. Tamulamides A and B were both found to compete with tritiated brevetoxin-3 ([(3)H]-PbTx-3) for its binding site on rat brain synaptosomes. However, unlike the brevetoxins, tamulamides A and B showed no toxicity to fish at doses up to 200 nM and did not cause significant bronchoconstriction in sheep pulmonary assays.
Figures

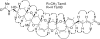
Similar articles
-
A new polyether ladder compound produced by the dinoflagellate Karenia brevis.J Nat Prod. 2005 Jan;68(1):2-6. doi: 10.1021/np049797o. J Nat Prod. 2005. PMID: 15679307 Free PMC article.
-
Brevisin: an aberrant polycyclic ether structure from the dinoflagellate Karenia brevis and its implications for polyether assembly.J Org Chem. 2009 Feb 6;74(3):989-94. doi: 10.1021/jo802183n. J Org Chem. 2009. PMID: 19123836
-
Brevisulcatic acids, marine ladder-frame polyethers from the red tide dinoflagellate Karenia brevisulcata in New Zealand.Org Lett. 2014 Nov 21;16(22):5850-3. doi: 10.1021/ol502700h. Epub 2014 Oct 30. Org Lett. 2014. PMID: 25356530
-
Recent advances in the synthesis of marine polycyclic ether natural products.Curr Opin Drug Discov Devel. 2007 Nov;10(6):784-806. Curr Opin Drug Discov Devel. 2007. PMID: 17987529 Review.
-
Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms.Mar Drugs. 2021 Nov 24;19(12):656. doi: 10.3390/md19120656. Mar Drugs. 2021. PMID: 34940655 Free PMC article. Review.
Cited by
-
Marine pharmacology in 2009-2011: marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action.Mar Drugs. 2013 Jul 16;11(7):2510-73. doi: 10.3390/md11072510. Mar Drugs. 2013. PMID: 23880931 Free PMC article. Review.
-
Both modular and single-domain Type I polyketide synthases are expressed in the brevetoxin-producing dinoflagellate, Karenia brevis (Dinophyceae).J Phycol. 2017 Dec;53(6):1325-1339. doi: 10.1111/jpy.12586. Epub 2017 Nov 3. J Phycol. 2017. PMID: 28949419 Free PMC article.
-
Catalytic Synthesis of 1H-2-Benzoxocins: Cobalt(III)-Carbene Radical Approach to 8-Membered Heterocyclic Enol Ethers.J Am Chem Soc. 2021 Dec 8;143(48):20501-20512. doi: 10.1021/jacs.1c10927. Epub 2021 Nov 21. J Am Chem Soc. 2021. PMID: 34802239 Free PMC article.
-
Profiling of Brevetoxin Metabolites Produced by Karenia brevis 165 Based on Liquid Chromatography-Mass Spectrometry.Toxins (Basel). 2021 May 14;13(5):354. doi: 10.3390/toxins13050354. Toxins (Basel). 2021. PMID: 34069292 Free PMC article.
-
Synthesis of the EFG Framework of Tamulamides A and B.Org Lett. 2019 Oct 4;21(19):8027-8030. doi: 10.1021/acs.orglett.9b03015. Epub 2019 Sep 16. Org Lett. 2019. PMID: 31523969 Free PMC article.
References
-
- Shimizu Y, Chou H-Y, Bando H. J. Am. Chem. Soc. 1986;1089:514–515. - PubMed
-
- Lin Y, Risk M, Ray SM, Van Engen D, Clardy J, Golik J, James JC, Nakanishi KJ. J. Am. Chem. Soc. 1981;103:6773–6775.
-
- Prasad AVK, Shimizu Y. J. Am. Chem. Soc. 1989;111:6476–6477.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

