Theory, instrumentation, and applications of electron paramagnetic resonance oximetry
- PMID: 20218670
- PMCID: PMC2868962
- DOI: 10.1021/cr900396q
Theory, instrumentation, and applications of electron paramagnetic resonance oximetry
Figures
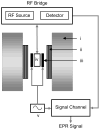

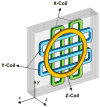
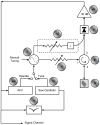

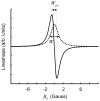
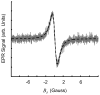
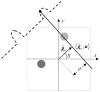
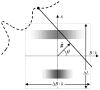
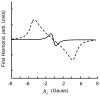

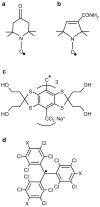
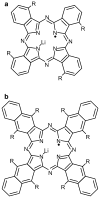
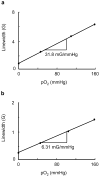
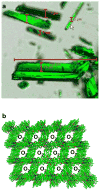
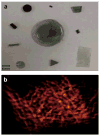
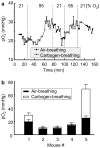
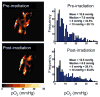
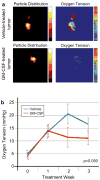
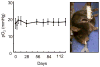
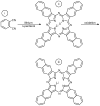
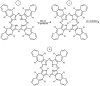
References
-
- Lane N. Oxygen: The Molecule that Made the World. Oxford University Press; Oxford: 2002.
-
- Falkowski PG. Science. 2006;311:1724. - PubMed
-
- Jiang YY, Kong DX, Qin T, Zhang HY. Biochem Biophys Res Commun. 2009 - PubMed
-
- Raymond J, Segre D. Science. 2006;311:1764. - PubMed
-
- Kulkarni AC, Kuppusamy P, Parinandi N. Antioxid Redox Signal. 2007;9:1717. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

