Systems analysis of protein modification and cellular responses induced by electrophile stress
- PMID: 20218676
- PMCID: PMC2873822
- DOI: 10.1021/ar900286y
Systems analysis of protein modification and cellular responses induced by electrophile stress
Abstract
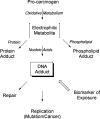
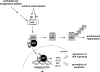
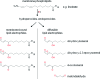
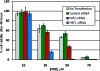
Biological electrophiles result from oxidative metabolism of exogenous compounds or endogenous cellular constituents, and they contribute to pathophysiologies such as toxicity and carcinogenicity. The chemical toxicology of electrophiles is dominated by covalent addition to intracellular nucleophiles. Reaction with DNA leads to the production of adducts that block replication or induce mutations. The chemistry and biology of electrophile-DNA reactions have been extensively studied, providing in many cases a detailed understanding of the relation between adduct structure and mutational consequences. By contrast, the linkage between protein modification and cellular response is poorly understood. In this Account, we describe our efforts to define the chemistry of protein modification and its biological consequences using lipid-derived alpha,beta-unsaturated aldehydes as model electrophiles. In our global approach, two large data sets are analyzed: one represents the identity of proteins modified over a wide range of electrophile concentrations, and the second comprises changes in gene expression observed under similar conditions. Informatics tools show theoretical connections based primarily on transcription factors hypothetically shared between the two data sets, downstream of adducted proteins and upstream of affected genes. This method highlights potential electrophile-sensitive signaling pathways and transcriptional processes for further evaluation. Peroxidation of cellular phospholipids generates a complex mixture of both membrane-bound and diffusible electrophiles. The latter include reactive species such as malondialdehyde, 4-oxononenal, and 4-hydroxynonenal (HNE). Enriching HNE-adducted proteins for proteomic analysis was a technical challenge, solved with click chemistry that generated biotin-tagged protein adducts. For this purpose, HNE analogues bearing terminal azide or alkyne functionalities were synthesized. Cellular lysates were first exposed to a single type of HNE analogue (azido- or alkynyl-HNE), and then click reactions were performed against the cognate alkynyl- and azido-biotin derivative. The resulting biotin-labeled proteins were captured and enriched over a streptavidin matrix for subsequent mass spectrometric analysis. We thereby identified a multitude of HNE targets. Simultaneous microarray analysis of changes in gene expression triggered by HNE also produced an abundance of data. Functional analysis of both data sets generated the hypothesis that an important pathway of cellular response derives from electrophile modification of protein chaperones, resulting in the release of transcription factors that are their clients. Informatic analysis of the protein modification and microarray data sets identified several transcription factors as potential mediators of the cellular response to HNE-adducted proteins. Among these, heat shock factor 1 (HSF1) was confirmed as a sensitive and robust effector of HNE-induced changes in gene expression. Activation of HSF1 appears, in part, to be mediated by the electrophilic adduction of Hsp70 and Hsp90, which normally maintain HSF1 in an inactive cytosolic complex. The identification of HSF1 as a mediator of biological effects downstream of HSF1 has provided new opportunities for research, illustrating the potential of our systems-based approach. Accordingly, we characterized HSF1-mediated gene expression in protecting against electrophile-induced toxicity. Among the genes induced by HSF1, Bcl-2- associated athanogene 3 (BAG3) is notable for its actions in promoting cell survival through stabilization of antiapoptotic Bcl-2 proteins, appearing to have a critical role in mediating cellular protection against electrophile-induced death.
Figures




Similar articles
-
Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL.J Biol Chem. 2007 Nov 16;282(46):33412-33420. doi: 10.1074/jbc.M706799200. Epub 2007 Sep 16. J Biol Chem. 2007. PMID: 17873279
-
Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives.Chem Res Toxicol. 2008 Feb;21(2):432-44. doi: 10.1021/tx700347w. Epub 2008 Jan 31. Chem Res Toxicol. 2008. PMID: 18232660 Free PMC article.
-
Measuring electrophile stress.Curr Protoc Toxicol. 2009;Chapter 17:Unit17.11. doi: 10.1002/0471140856.tx1711s40. Curr Protoc Toxicol. 2009. PMID: 23045010
-
Endogenous glutathione adducts.Curr Drug Metab. 2006 Dec;7(8):853-72. doi: 10.2174/138920006779010601. Curr Drug Metab. 2006. PMID: 17168687 Review.
-
Reactions of electrophiles with nucleophilic thiolate sites: relevance to pathophysiological mechanisms and remediation.Free Radic Res. 2016;50(2):195-205. doi: 10.3109/10715762.2015.1094184. Epub 2015 Nov 11. Free Radic Res. 2016. PMID: 26559119 Free PMC article. Review.
Cited by
-
Chemical reactivities of ambient air samples in three Southern California communities.J Air Waste Manag Assoc. 2015 Mar;65(3):270-7. doi: 10.1080/10962247.2014.988307. J Air Waste Manag Assoc. 2015. PMID: 25947123 Free PMC article.
-
Biochemical alterations in the oocyte in support of early embryonic development.Cell Mol Life Sci. 2017 Feb;74(3):469-485. doi: 10.1007/s00018-016-2356-1. Epub 2016 Sep 7. Cell Mol Life Sci. 2017. PMID: 27604868 Free PMC article. Review.
-
Chalcone: A Privileged Structure in Medicinal Chemistry.Chem Rev. 2017 Jun 28;117(12):7762-7810. doi: 10.1021/acs.chemrev.7b00020. Epub 2017 May 10. Chem Rev. 2017. PMID: 28488435 Free PMC article. Review.
-
Sulphoxythiocarbamates modify cysteine residues in HSP90 causing degradation of client proteins and inhibition of cancer cell proliferation.Br J Cancer. 2014 Jan 7;110(1):71-82. doi: 10.1038/bjc.2013.710. Epub 2013 Dec 5. Br J Cancer. 2014. PMID: 24322890 Free PMC article.
-
Redox regulation by reversible protein S-thiolation in bacteria.Front Microbiol. 2015 Mar 16;6:187. doi: 10.3389/fmicb.2015.00187. eCollection 2015. Front Microbiol. 2015. PMID: 25852656 Free PMC article. Review.
References
-
- Rossi A.; Kapahi P.; Natoli G.; Takahashi T.; Chen Y.; Karin M.; Santoro M. G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 2000, 403, 103–108. - PubMed
-
- Ji C.; Kozak K. R.; Marnett L. J. IκB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001, 276, 18223–1828. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

