Differential inhibition of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and TZM-bl cells by endotoxin-mediated chemokine and gamma interferon production
- PMID: 20218881
- PMCID: PMC2864054
- DOI: 10.1089/aid.2009.0186
Differential inhibition of human immunodeficiency virus type 1 in peripheral blood mononuclear cells and TZM-bl cells by endotoxin-mediated chemokine and gamma interferon production
Abstract
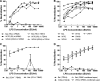
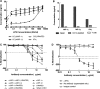
Bacterial lipopolysaccharide (endotoxin) is a frequent contaminant of biological specimens and is also known to be a potent inducer of beta-chemokines and other soluble factors that inhibit HIV-1 infection in vitro. Though lipopolysaccharide (LPS) has been shown to stimulate the production of soluble HIV-1 inhibitors in cultures of monocyte-derived macrophages, the ability of LPS to induce similar inhibitors in other cell types is poorly characterized. Here we show that LPS exhibits potent anti-HIV activity in phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMCs) but has no detectable anti-HIV-1 activity in TZM-bl cells. The anti-HIV-1 activity of LPS in PBMCs was strongly associated with the production of beta-chemokines from CD14-positive monocytes. Culture supernatants from LPS-stimulated PBMCs exhibited potent anti-HIV-1 activity when added to TZM-bl cells but, in this case, the antiviral activity appeared to be related to IFN-gamma rather than to beta-chemokines. These observations indicate that LPS stimulates PBMCs to produce a complex array of soluble HIV-1 inhibitors, including beta-chemokines and IFN-gamma, that differentially inhibit HIV-1 depending on the target cell type. The results also highlight the need to use endotoxin-free specimens to avoid artifacts when assessing HIV-1-specific neutralizing antibodies in PBMC-based assays.
Figures
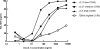

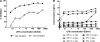
Similar articles
-
Divergent effects of cell environment on HIV entry inhibitor activity.AIDS. 2009 Jul 17;23(11):1319-27. doi: 10.1097/qad.0b013e32832d92c2. AIDS. 2009. PMID: 19579289
-
Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to That of Nonneutralizing Antibodies.J Virol. 2016 Dec 16;91(1):e01762-16. doi: 10.1128/JVI.01762-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795431 Free PMC article.
-
Circulating levels and ex vivo production of beta-chemokines, interferon gamma, and interleukin 2 in advanced human immunodeficiency virus type 1 infection: the effect of protease inhibitor therapy.AIDS Res Hum Retroviruses. 2000 Jun 10;16(9):835-43. doi: 10.1089/08892220050042774. AIDS Res Hum Retroviruses. 2000. PMID: 10875609 Clinical Trial.
-
Mannoprotein-induced anti-U937 cell cytotoxicity in peripheral blood mononuclear cells from uninfected or HIV-infected subjects: role of interferon-gamma and tumor necrosis factor-alpha.Cell Immunol. 1993 Dec;152(2):530-43. doi: 10.1006/cimm.1993.1310. Cell Immunol. 1993. PMID: 8258154
-
Human peripheral blood T cells, monocytes, and macrophages secrete macrophage inflammatory proteins 1alpha and 1beta following stimulation with heat-inactivated Brucella abortus.Infect Immun. 2001 Jun;69(6):3817-26. doi: 10.1128/IAI.69.6.3817-3826.2001. Infect Immun. 2001. PMID: 11349047 Free PMC article.
Cited by
-
Immunization with hybrid proteins containing the membrane proximal external region of HIV-1.AIDS Res Hum Retroviruses. 2014 May;30(5):498-508. doi: 10.1089/AID.2013.0191. Epub 2014 Feb 7. AIDS Res Hum Retroviruses. 2014. PMID: 24392780 Free PMC article.
-
Identification and characterization of a broadly cross-reactive HIV-1 human monoclonal antibody that binds to both gp120 and gp41.PLoS One. 2012;7(9):e44241. doi: 10.1371/journal.pone.0044241. Epub 2012 Sep 10. PLoS One. 2012. PMID: 22970187 Free PMC article.
-
Assessment of Antibody Interference of Enfuvirtide (T20) Function Shows Assay Dependent Variability.Curr HIV Res. 2018;16(6):404-415. doi: 10.2174/1570162X17666190228154850. Curr HIV Res. 2018. PMID: 30836922 Free PMC article.
-
The Pre-clinical Toolbox of Pharmacokinetics and Pharmacodynamics: in vitro and ex vivo Models.Front Pharmacol. 2019 May 24;10:578. doi: 10.3389/fphar.2019.00578. eCollection 2019. Front Pharmacol. 2019. PMID: 31178736 Free PMC article. Review.
-
Antibodies to a superantigenic glycoprotein 120 epitope as the basis for developing an HIV vaccine.J Immunol. 2012 Dec 1;189(11):5367-81. doi: 10.4049/jimmunol.1200981. Epub 2012 Oct 22. J Immunol. 2012. PMID: 23089396 Free PMC article.
References
-
- Polonis VR. Brown BK. Borges AR, et al. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology. 2008;375:315–320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

