Modulation of ColE1-like plasmid replication for recombinant gene expression
- PMID: 20218961
- PMCID: PMC2846101
- DOI: 10.2174/187221510790410822
Modulation of ColE1-like plasmid replication for recombinant gene expression
Abstract
ColE1-like plasmids constitute the most popular vectors for recombinant protein expression. ColE1 plasmid replication is tightly controlled by an antisense RNA mechanism that is highly dynamic, tuning plasmid metabolic burden to the physiological state of the host. Plasmid homeostasis is upset upon induction of recombinant protein expression because of non-physiological levels of expression and because of the frequently biased amino acid composition of recombinant proteins. Disregulation of plasmid replication is the main cause of collapse of plasmid-based expression systems because of a simultaneous increase in the metabolic burden (due to increased average copy number) and in the probability of generation of plasmid-free cells (due to increased copy number variation). Interference between regulatory elements of co-resident plasmids causes comparable effects on plasmid stability (plasmid incompatibility). Modulating plasmid copy number for recombinant gene expression aims at achieving a high gene dosage while preserving the stability of the expression system. Here I present strategies targeting plasmid replication for optimizing recombinant gene expression. Specifically, I review approaches aimed at modulating the antisense regulatory system (as well as their implications for plasmid incompatibility) and innovative strategies involving modulation of host factors, of R-loop formation, and of the timing of recombinant gene expression.
Conflict of interest statement
The author has no conflicts of interest that are directly relevant to the contents of this manuscript
Figures
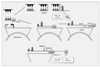


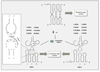


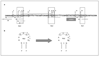

Similar articles
-
Analysis of establishment phase replication of the plasmid ColE1.J Mol Biol. 1993 Mar 5;230(1):137-50. doi: 10.1006/jmbi.1993.1131. J Mol Biol. 1993. PMID: 7680724
-
Characterization of pEC156, a ColE1-type plasmid from Escherichia coli E1585-68 that carries genes of the EcoVIII restriction-modification system.Plasmid. 2001 Sep;46(2):128-39. doi: 10.1006/plas.2001.1534. Plasmid. 2001. PMID: 11591138
-
Impact of targeted vector design on Co/E1 plasmid replication.Trends Biotechnol. 2002 Jun;20(6):257-60. doi: 10.1016/s0167-7799(02)01950-9. Trends Biotechnol. 2002. PMID: 12007494 Review.
-
The TraM protein of the conjugative plasmid F binds to the origin of transfer of the F and ColE1 plasmids.Mol Microbiol. 1992 Oct;6(20):2951-9. doi: 10.1111/j.1365-2958.1992.tb01754.x. Mol Microbiol. 1992. PMID: 1479887
-
Control of ColE1 plasmid replication by antisense RNA.Trends Genet. 1991 Jul;7(7):230-5. doi: 10.1016/0168-9525(91)90370-6. Trends Genet. 1991. PMID: 1887504 Review.
Cited by
-
A degenerate primer MOB typing (DPMT) method to classify gamma-proteobacterial plasmids in clinical and environmental settings.PLoS One. 2012;7(7):e40438. doi: 10.1371/journal.pone.0040438. Epub 2012 Jul 11. PLoS One. 2012. PMID: 22792321 Free PMC article.
-
Reducing metabolic burden in the PACEmid evolver system by remastering high-copy phagemid vectors.Eng Biol. 2022 May 20;6(2-3):50-61. doi: 10.1049/enb2.12021. eCollection 2022 Jun-Sep. Eng Biol. 2022. PMID: 36969104 Free PMC article.
-
Boundaries of the origin of replication: creation of a pET-28a-derived vector with p15A copy control allowing compatible coexistence with pET vectors.PLoS One. 2012;7(10):e47259. doi: 10.1371/journal.pone.0047259. Epub 2012 Oct 22. PLoS One. 2012. PMID: 23110063 Free PMC article.
-
Stochastic nature and physiological implications of 5'-NAD RNA cap in bacteria.Nucleic Acids Res. 2024 Oct 28;52(19):11838-11852. doi: 10.1093/nar/gkae813. Nucleic Acids Res. 2024. PMID: 39325642 Free PMC article.
-
Development of high-copy number plasmids in Pseudoalteromonas haloplanktis TAC125.Appl Microbiol Biotechnol. 2023 Apr;107(7-8):2469-2481. doi: 10.1007/s00253-023-12448-w. Epub 2023 Mar 13. Appl Microbiol Biotechnol. 2023. PMID: 36912903 Free PMC article.
References
-
- Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. - PubMed
-
- Balbas P, Bolivar F. Back to basics: pBR322 and protein expression systems in E. coli. Methods Mol Biol. 2004;267:77–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

