The metabolic enhancer piracetam ameliorates the impairment of mitochondrial function and neurite outgrowth induced by beta-amyloid peptide
- PMID: 20218980
- PMCID: PMC2874847
- DOI: 10.1111/j.1476-5381.2010.00656.x
The metabolic enhancer piracetam ameliorates the impairment of mitochondrial function and neurite outgrowth induced by beta-amyloid peptide
Abstract
Background and purpose: beta-Amyloid peptide (Abeta) is implicated in the pathogenesis of Alzheimer's disease by initiating a cascade of events from mitochondrial dysfunction to neuronal death. The metabolic enhancer piracetam has been shown to improve mitochondrial dysfunction following brain aging and experimentally induced oxidative stress.
Experimental approach: We used cell lines (PC12 and HEK cells) and murine dissociated brain cells. The protective effects of piracetam in vitro and ex vivo on Abeta-induced impairment of mitochondrial function (as mitochondrial membrane potential and ATP production), on secretion of soluble Abeta and on neurite outgrowth in PC12 cells were investigated.
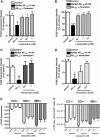
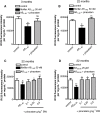
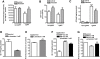
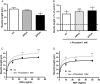
Key results: Piracetam improves mitochondrial function of PC12 cells and acutely dissociated brain cells from young NMRI mice following exposure to extracellular Abeta(1-42). Similar protective effects against Abeta(1-42) were observed in dissociated brain cells from aged NMRI mice, or mice transgenic for mutant human amyloid precursor protein (APP) treated with piracetam for 14 days. Soluble Abeta load was markedly diminished in the brain of those animals after treatment with piracetam. Abeta production by HEK cells stably transfected with mutant human APP was elevated by oxidative stress and this was reduced by piracetam. Impairment of neuritogenesis is an important consequence of Abeta-induced mitochondrial dysfunction and Abeta-induced reduction of neurite growth in PC12 cells was substantially improved by piracetam.
Conclusion and implications: Our findings strongly support the concept of improving mitochondrial function as an approach to ameliorate the detrimental effects of Abeta on brain function.
Figures

Comment in
-
Mitochondria as pharmacological targets.Br J Pharmacol. 2010 May;160(2):217-9. doi: 10.1111/j.1476-5381.2010.00706.x. Br J Pharmacol. 2010. PMID: 20423336 Free PMC article.
Similar articles
-
Piracetam improves mitochondrial dysfunction following oxidative stress.Br J Pharmacol. 2006 Jan;147(2):199-208. doi: 10.1038/sj.bjp.0706459. Br J Pharmacol. 2006. PMID: 16284628 Free PMC article.
-
Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid beta-peptide (1-42), HO and kainic acid: implications for Alzheimer's disease.J Neurochem. 2006 Mar;96(5):1322-35. doi: 10.1111/j.1471-4159.2005.03647.x. J Neurochem. 2006. PMID: 16478525
-
Protective effects of [Gly14]-Humanin on beta-amyloid-induced PC12 cell death by preventing mitochondrial dysfunction.Neurochem Int. 2010 Feb;56(3):417-23. doi: 10.1016/j.neuint.2009.11.015. Epub 2009 Nov 24. Neurochem Int. 2010. PMID: 19941922
-
[Search of Neurotrophin-mimic Natural Products for Prevention and Treatment of Neurodegenerative Disease].Yakugaku Zasshi. 2015;135(10):1147-52. doi: 10.1248/yakushi.15-00197. Yakugaku Zasshi. 2015. PMID: 26423871 Review. Japanese.
-
The role of amyloid-beta in the regulation of memory.Biochem Pharmacol. 2014 Apr 15;88(4):479-85. doi: 10.1016/j.bcp.2013.12.018. Epub 2014 Jan 4. Biochem Pharmacol. 2014. PMID: 24398426 Review.
Cited by
-
Enhanced Neuroplasticity by the Metabolic Enhancer Piracetam Associated with Improved Mitochondrial Dynamics and Altered Permeability Transition Pore Function.Neural Plast. 2016;2016:8075903. doi: 10.1155/2016/8075903. Epub 2016 Sep 26. Neural Plast. 2016. PMID: 27747106 Free PMC article.
-
Hemp (Cannabis sativa L.) Seed Phenylpropionamides Composition and Effects on Memory Dysfunction and Biomarkers of Neuroinflammation Induced by Lipopolysaccharide in Mice.ACS Omega. 2018 Nov 30;3(11):15988-15995. doi: 10.1021/acsomega.8b02250. Epub 2018 Nov 27. ACS Omega. 2018. PMID: 30556022 Free PMC article.
-
Neuroprotective effect of novel cognitive enhancer noopept on AD-related cellular model involves the attenuation of apoptosis and tau hyperphosphorylation.J Biomed Sci. 2014 Aug 6;21(1):74. doi: 10.1186/s12929-014-0074-2. J Biomed Sci. 2014. PMID: 25096780 Free PMC article.
-
Galangin's Neuroprotective Role: Targeting Oxidative Stress, Inflammation, and Apoptosis in Ischemic Stroke in a Rat Model of Permanent Middle Cerebral Artery Occlusion.Int J Mol Sci. 2025 Feb 21;26(5):1847. doi: 10.3390/ijms26051847. Int J Mol Sci. 2025. PMID: 40076473 Free PMC article.
-
Mitochondrial dysfunction: common final pathway in brain aging and Alzheimer's disease--therapeutic aspects.Mol Neurobiol. 2010 Jun;41(2-3):159-71. doi: 10.1007/s12035-010-8141-5. Epub 2010 May 12. Mol Neurobiol. 2010. PMID: 20461558 Review.
References
-
- Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–712. - PubMed
-
- Bachurin SA, Shevtsova EP, Kireeva EG, Oxebkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003;993:334–344. - PubMed
-
- Benzi G, Pastoris O, Villa RR, Giuffrida AM. Influence of aging and exogenous substances on cerebral energy metabolism in posthypoglycemic recovery. Biochem Pharmacol. 1985;34:1477–1483. - PubMed
-
- Bernales S, Wagner S, Protter AA, Hung DT. Dimebon induces neurite outgrowth and mitochondrial statilizion. Washington, DC: Society for Neuroscience; 2008. Program No. 543.29/S12. Neuroscience Meeting Planner. Online.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

