Imaging and biodistribution of Her2/neu expression in non-small cell lung cancer xenografts with Cu-labeled trastuzumab PET
- PMID: 20219072
- PMCID: PMC11159917
- DOI: 10.1111/j.1349-7006.2010.01480.x
Imaging and biodistribution of Her2/neu expression in non-small cell lung cancer xenografts with Cu-labeled trastuzumab PET
Abstract
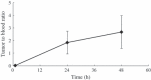
Non-small cell lung carcinomas (NSCLC) overexpress the Her2/neu gene in approximately 59% of cases. Trastuzumab, a humanized monoclonal antibody, interferes with Her2 signaling and is approved for the treatment of Her2/neu overexpressing breast cancer. However, its therapeutic use in Her2/neu overexpressing NSCLC remains obscure. The present study aimed to determine the role of (64)Cu-labeled trastuzumab positron emission tomography (PET) for non-invasive imaging of Her2/neu expression in NSCLC. Trastuzumab was conjugated with the bifunctional chelator 1, 4, 7, 10-tetraazacyclododecane-1, 4, 7, 10-tetraacetic acid (DOTA) and radiolabeled with (64)Cu. The molecular specificity of DOTA-trastuzumab was determined in NSCLC cell lines with Her2/neu overexpression (NCI-H2170) and negative expression (NCI-H520). Imaging of Her2/neu expression was performed in NCI-H2170 tumor-bearing mice with (64)Cu-DOTA-trastuzumab PET and (64)Cu-DOTA-IgG. In vitro studies revealed specific binding of DOTA-trastuzumab in the Her2/neu positive NCI-H2170 cells, while no binding was seen in the Her2/neu negative NCI-H520 cell line. Biodistribution and PET studies revealed a significantly high accumulation of (64)Cu-DOTA-trastuzumab in the Her2/neu overexpressing NCI-H2170 tumor at 24 and 48 h post-injection (21.4 +/- 1.4% and 23.2 +/- 5.1% injection dose/gram (% ID/g), respectively). PET imaging of Her2/neu negative NCI-H520 tumors showed much less uptake of (64)Cu-DOTA-trastuzumab (4.0% ID/g). The NCI-H2170 tumor uptake of (64)Cu-DOTA-trastuzumab was significantly higher than that of (64)Cu-DOTA-IgG (P < 0.0001). (64)Cu-DOTA-trastuzumab showed a very clear image of a Her2/neu positive tumor and appeared to be effective as a PET tracer for imaging of Her2/neu gene expression in NSCLC, suggesting its potential clinical use for identifying patients that might benefit from trastuzumab-based therapy.
Conflict of interest statement
None declared by all authors.
Figures




Similar articles
-
Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using (64)Cu-DOTA-trastuzumab PET.J Nucl Med. 2014 Jan;55(1):23-9. doi: 10.2967/jnumed.113.122630. Epub 2013 Dec 12. J Nucl Med. 2014. PMID: 24337604 Free PMC article.
-
Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging.J Nucl Med. 2009 Jun;50(6):974-81. doi: 10.2967/jnumed.108.060392. Epub 2009 May 14. J Nucl Med. 2009. PMID: 19443585
-
68Ga-DOTA-affibody molecule for in vivo assessment of HER2/neu expression with PET.Eur J Nucl Med Mol Imaging. 2011 Nov;38(11):1967-76. doi: 10.1007/s00259-011-1810-4. Epub 2011 Apr 20. Eur J Nucl Med Mol Imaging. 2011. PMID: 21748382 Free PMC article.
-
64Cu-1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid-human serum albumin-Ac-Cys-ZEGFR:1907.2012 Jun 25 [updated 2012 Sep 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Jun 25 [updated 2012 Sep 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23035309 Free Books & Documents. Review.
-
Preclinical studies of gemcitabine and trastuzumab in breast and lung cancer cell lines.Clin Breast Cancer. 2002 May;3 Suppl 1:12-6. doi: 10.3816/cbc.2002.s.003. Clin Breast Cancer. 2002. PMID: 12057039 Review.
Cited by
-
Controlled administration of penicillamine reduces radiation exposure in critical organs during 64Cu-ATSM internal radiotherapy: a novel strategy for liver protection.PLoS One. 2014 Jan 22;9(1):e86996. doi: 10.1371/journal.pone.0086996. eCollection 2014. PLoS One. 2014. PMID: 24466309 Free PMC article.
-
Predicting cetuximab accumulation in KRAS wild-type and KRAS mutant colorectal cancer using 64Cu-labeled cetuximab positron emission tomography.Cancer Sci. 2012 Mar;103(3):600-5. doi: 10.1111/j.1349-7006.2011.02166.x. Epub 2011 Dec 23. Cancer Sci. 2012. PMID: 22126621 Free PMC article.
-
PET imaging with ⁸⁹Zr: from radiochemistry to the clinic.Nucl Med Biol. 2013 Jan;40(1):3-14. doi: 10.1016/j.nucmedbio.2012.08.004. Epub 2012 Sep 19. Nucl Med Biol. 2013. PMID: 22998840 Free PMC article. Review.
-
Observations on the Effects of Residualization and Dehalogenation on the Utility of N-Succinimidyl Ester Acylation Agents for Radioiodination of the Internalizing Antibody Trastuzumab.Molecules. 2019 Oct 30;24(21):3907. doi: 10.3390/molecules24213907. Molecules. 2019. PMID: 31671554 Free PMC article.
-
Radiochemistry, Production Processes, Labeling Methods, and ImmunoPET Imaging Pharmaceuticals of Iodine-124.Molecules. 2021 Jan 14;26(2):414. doi: 10.3390/molecules26020414. Molecules. 2021. PMID: 33466827 Free PMC article. Review.
References
-
- Micheli A, Mugno E, Krogh V et al. Cancer prevalence in European registry areas. Ann Oncol 2002; 13: 840–65. - PubMed
-
- Tsai CM, Chang KT, Perng RP et al. Correlation of intrinsic chemoresistance of non‐small cell lung cancer cell lines with HER‐2/neu gene expression but not with ras gene mutations. J Natl Cancer Inst 1993; 85: 897–901. - PubMed
-
- Garrett TP, McKern NM, Lou M et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 2003; 11: 495–505. - PubMed
-
- Baselga J, Norton L, Albanell J et al. Recombinant humanized anti‐HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58: 2825–31. - PubMed
-
- Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false‐positives do not get the message. J Clin Oncol 2001; 19: 2714–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

