Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer
- PMID: 20219073
- PMCID: PMC11159155
- DOI: 10.1111/j.1349-7006.2010.01492.x
Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer
Abstract
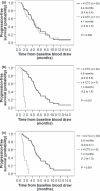
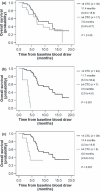
The purpose of this study was to quantify circulating tumor cells (CTCs) in advanced gastric cancer (AGC) patients, and to demonstrate the role of CTCs in cancer therapy. This study investigates the hypothesis that CTCs can predict clinical outcomes in patients with AGC. From November 2007 to June 2009, 52 patients with AGC were enrolled into a prospective study. The chemotherapy regimen was an S-1-based regimen (S-1 with or without cisplatin) or paclitaxel. CTCs of whole blood at baseline, 2 weeks, and 4 weeks after initiation of chemotherapy, were isolated and enumerated using immunomagnetics. Patients with > or =4 CTCs at 2-week points and 4-week points had a shorter median progression-free survival (PFS) (1.4, 1.4 months, respectively) than those with the median PFS of <4 CTCs (4.9, 5.0 months, respectively) (log-rank test; P < 0.001, P < 0.001, respectively). Patients with > or =4 CTCs at 2-week points and 4-week points had shorter median overall survival (OS) (3.5, 4.0 months, respectively) than those with the median PFS of <4 CTCs (11.7, 11.4 months, respectively) (log-rank test; P < 0.001, P = 0.001, respectively). In conclusion, this study demonstrates that CTC measurement may be useful as a surrogate marker for determining response to S-1-based or paclitaxel regimens in AGC.
Figures
Similar articles
-
[Efficacy of lobaplatin plus S-1 and the predictive value of circulating tumor cell in patients with advanced gastric cancer].Zhonghua Zhong Liu Za Zhi. 2018 Sep 23;40(9):696-702. doi: 10.3760/cma.j.issn.0253-3766.2018.09.012. Zhonghua Zhong Liu Za Zhi. 2018. PMID: 30293397 Chinese.
-
Dynamic monitoring of circulating tumour cells to evaluate therapeutic efficacy in advanced gastric cancer.Br J Cancer. 2016 Jan 19;114(2):138-45. doi: 10.1038/bjc.2015.417. Br J Cancer. 2016. PMID: 26784122 Free PMC article.
-
Phase II multi-institutional prospective randomised trial comparing S-1+paclitaxel with S-1+cisplatin in patients with unresectable and/or recurrent advanced gastric cancer.Br J Cancer. 2012 Jun 26;107(1):31-6. doi: 10.1038/bjc.2012.222. Epub 2012 May 22. Br J Cancer. 2012. PMID: 22617130 Free PMC article. Clinical Trial.
-
S-1 combined with paclitaxel may benefit advanced gastric cancer: Evidence from a systematic review and meta-analysis.Int J Surg. 2019 Feb;62:34-43. doi: 10.1016/j.ijsu.2018.11.010. Epub 2019 Jan 12. Int J Surg. 2019. PMID: 30641155
-
S-1-based combination therapy vs S-1 monotherapy in advanced gastric cancer: a meta-analysis.World J Gastroenterol. 2014 Jan 7;20(1):310-8. doi: 10.3748/wjg.v20.i1.310. World J Gastroenterol. 2014. PMID: 24415887 Free PMC article. Review.
Cited by
-
High circulating tumor cell concentrations in a specific subtype of gastric cancer with diffuse bone metastasis at diagnosis.World J Gastroenterol. 2016 Jul 14;22(26):6083-8. doi: 10.3748/wjg.v22.i26.6083. World J Gastroenterol. 2016. PMID: 27468200 Free PMC article.
-
Liquid biopsy in gastric cancer: predictive and prognostic biomarkers.Cell Death Dis. 2022 Oct 27;13(10):903. doi: 10.1038/s41419-022-05350-2. Cell Death Dis. 2022. PMID: 36302755 Free PMC article. Review.
-
Moving forward with circulating tumor cells and lung cancer.J Thorac Dis. 2012 Oct;4(5):440-1. doi: 10.3978/j.issn.2072-1439.2012.08.08. J Thorac Dis. 2012. PMID: 23050099 Free PMC article. No abstract available.
-
Prognostic value of circulating tumor cells detected with the CellSearch System in patients with gastric cancer: evidence from a meta-analysis.Onco Targets Ther. 2018 Feb 26;11:1013-1023. doi: 10.2147/OTT.S154114. eCollection 2018. Onco Targets Ther. 2018. PMID: 29520152 Free PMC article.
-
Clinical significance of circulating tumor cells in gastric cancer patients.Oncotarget. 2017 Apr 11;8(15):25713-25720. doi: 10.18632/oncotarget.14879. Oncotarget. 2017. PMID: 28147337 Free PMC article. Review.
References
-
- Murad AM, Santiago FF, Petroianu A, Rocha PRS, Rodrigues MAG, Rauusch M. Modified therapy with 5‐fluorouracil, doxorubicine, and methotrexate in advanced gastric cancer. Cancer 1993; 72: 37–41. - PubMed
-
- Glimelius B, Hoffman K, Haglund U, Nyren O, Sjoden PO. Initial or delayed chemotherapy with best supportive care in advanced gastric cancer. Ann Oncol 1994; 5: 189–90. - PubMed
-
- Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer 2004; 4: 448–56. - PubMed
-
- Ring A, Smithe IE, Dowsett M. Circulating tumour cells in breast cancer. Lancet Oncol 2004; 5: 79–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

