Suppressive effect of azithromycin on Plasmodium berghei mosquito stage development and apicoplast replication
- PMID: 20219090
- PMCID: PMC2846956
- DOI: 10.1186/1475-2875-9-73
Suppressive effect of azithromycin on Plasmodium berghei mosquito stage development and apicoplast replication
Abstract
Background: Azithromycin (AZM) is a macrolide antibiotic that displays an excellent safety profile even in children and pregnant women and has been shown to have anti-malarial activity against blood stage Plasmodium falciparum. This study evaluated the transmission-blocking effect of AZM using a rodent malaria model.
Methods: AZM-treated mice infected with Plasmodium berghei were exposed to Anopheles stephensi mosquitoes, followed by the observation of parasite development at different phases in the mosquito, i.e., ookinetes in the midgut, oocysts on the midgut, and sporozoites in the midgut and salivary glands. Furthermore, to evaluate the effect on organelle replication of each stage, quantitative real-time PCR analysis was performed.
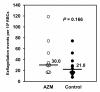
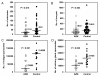
Results: The inhibitory effect of AZM was noticeable in both gametocyte-ookinete transformation in the midgut and sporozoite production in the oocyst, while the latter was most remarkable among all the developmental phases examined. Real-time PCR analysis revealed that AZM suppressed apicoplast replication at the period of sporozoite production in oocysts.
Conclusions: AZM inhibits parasite development in the mosquito stage, probably through the same mechanism as in the liver and blood stages. Such a multi-targeting anti-malarial, along with its safety, would be ideal for mass drug administration in malaria control programmes.
Figures

References
-
- Aregawi M, Cibulskis R, Otten M, Williams R, Dye C. World Malaria Report 2008. World Health Organization, Geneva; 2008.
-
- Coleman RE, Nath AK, Schneider I, Song GH, Klein TA, Milhous WK. Prevention of sporogony of Plasmodium falciparum and P. berghei in Anopheles stephensi mosquitoes by transmission-blocking antimalarials. Am J Trop Med Hyg. 1994;50:646–653. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

