Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development
- PMID: 20219117
- PMCID: PMC2845137
- DOI: 10.1186/1477-7827-8-23
Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development
Abstract
Background: Adiponectin is an adipokine, mainly produced by adipose tissue. It regulates several reproductive processes. The protein expression of the adiponectin system (adiponectin, its receptors, AdipoR1 and AdipoR2 and the APPL1 adaptor) in bovine ovary and its role on ovarian cells and embryo, remain however to be determined.
Methods: Here, we identified the adiponectin system in bovine ovarian cells and embryo using RT-PCR, immunoblotting and immunohistochemistry. Furthermore, we investigated in vitro the effects of recombinant human adiponectin (10 micro g/mL) on proliferation of granulosa cells (GC) measured by [3H] thymidine incorporation, progesterone and estradiol secretions measured by radioimmunoassay in the culture medium of GC, nuclear oocyte maturation and early embryo development.
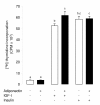
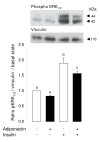
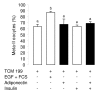
Results: We show that the mRNAs and proteins for the adiponectin system are present in bovine ovary (small and large follicles and corpus luteum) and embryo. Adiponectin, AdipoR1 and AdipoR2 were more precisely localized in oocyte, GC and theca cells. Adiponectin increased IGF-1 10(-8) M-induced GC proliferation (P < 0.01) but not basal or insulin 10(-8) M-induced proliferation. Additionally, adiponectin decreased insulin 10(-8) M-induced, but not basal or IGF-1 10(-8) M-induced secretions of progesterone (P < 0.01) and estradiol (P < 0.05) by GC. This decrease in insulin-induced steroidogenesis was associated with a decrease in ERK1/2 MAPK phosphorylation in GC pre-treated with adiponectin. Finally, addition of adiponectin during in vitro maturation affected neither the percentage of oocyte in metaphase-II nor 48-h cleavage and blastocyst day 8 rates.
Conclusions: In bovine species, adiponectin decreased insulin-induced steroidogenesis and increased IGF-1-induced proliferation of cultured GC through a potential involvement of ERK1/2 MAPK pathway, whereas it did not modify oocyte maturation and embryo development in vitro.
Figures


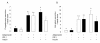



Similar articles
-
Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis.Reproduction. 2007 Apr;133(4):719-31. doi: 10.1530/REP-06-0244. Reproduction. 2007. PMID: 17504916
-
Adiponectin increases insulin-like growth factor I-induced progesterone and estradiol secretion in human granulosa cells.Fertil Steril. 2009 Dec;92(6):1988-96. doi: 10.1016/j.fertnstert.2008.09.008. Epub 2008 Dec 10. Fertil Steril. 2009. PMID: 19081562
-
Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells.Reproduction. 2017 May;153(5):589-603. doi: 10.1530/REP-16-0677. Epub 2017 Mar 1. Reproduction. 2017. PMID: 28250234
-
Adiponectin and the control of female reproductive functions.Vitam Horm. 2012;90:239-87. doi: 10.1016/B978-0-12-398313-8.00010-5. Vitam Horm. 2012. PMID: 23017719 Review.
-
Mechanisms of Adiponectin Action in Fertility: An Overview from Gametogenesis to Gestation in Humans and Animal Models in Normal and Pathological Conditions.Int J Mol Sci. 2019 Mar 27;20(7):1526. doi: 10.3390/ijms20071526. Int J Mol Sci. 2019. PMID: 30934676 Free PMC article. Review.
Cited by
-
Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human.Fertil Steril. 2012 Aug;98(2):471-9.e1. doi: 10.1016/j.fertnstert.2012.04.050. Epub 2012 May 26. Fertil Steril. 2012. PMID: 22633650 Free PMC article.
-
Impact of the severity of negative energy balance on gene expression in the subcutaneous adipose tissue of periparturient primiparous Holstein dairy cows: Identification of potential novel metabolic signals for the reproductive system.PLoS One. 2019 Sep 26;14(9):e0222954. doi: 10.1371/journal.pone.0222954. eCollection 2019. PLoS One. 2019. PMID: 31557215 Free PMC article.
-
Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds.Int J Mol Sci. 2020 May 19;21(10):3581. doi: 10.3390/ijms21103581. Int J Mol Sci. 2020. PMID: 32438614 Free PMC article. Review.
-
Role of vaspin in porcine ovary: effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A†.Biol Reprod. 2020 May 26;102(6):1290-1305. doi: 10.1093/biolre/ioaa027. Biol Reprod. 2020. PMID: 32149334 Free PMC article.
-
Localization and expression of C1QTNF6 in chicken follicles and its regulatory effect on follicular granulosa cells.Poult Sci. 2025 Jan;104(1):104538. doi: 10.1016/j.psj.2024.104538. Epub 2024 Nov 8. Poult Sci. 2025. PMID: 39566174 Free PMC article.
References
-
- Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

