Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo
- PMID: 20219180
- PMCID: PMC2880164
- DOI: 10.1016/j.mvr.2010.03.001
Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo
Abstract
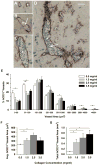
Developing tissue engineering approaches to generate functional vascular networks is important for improving treatments of peripheral and cardiovascular disease. Endothelial colony forming cells (ECFCs) are an endothelial progenitor cell (EPC) population defined by high proliferative potential and an ability to vascularize collagen-based matrices in vivo. Little is known regarding how physical properties of the local cell microenvironment guide vessel formation following EPC transplantation. In vitro evidence suggests that collagen matrix stiffness may modulate EPC vessel formation. The present study determined the ability of 3D collagen matrix physical properties, varied by changing collagen concentration, to influence ECFC vasculogenesis in vivo. Human umbilical cord blood ECFCs were cultured within matrices for 18 h in vitro and then fixed for in vitro analysis or implanted subcutaneously into the flank of immunodeficient mice for 14 days. We report that increasing collagen concentration significantly decreased ECFC derived vessels per area (density), but significantly increased vessel sizes (total cross sectional area). These results demonstrate that the physical properties of collagen matrices influence ECFC vasculogenesis in vivo and that by modulating these properties, one can guide vascularization.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Abraham LC, Zuena E, et al. Guide to collagen characterization for biomaterial studies. J Biomed Mater Res B Appl Biomater. 2008;87(1):264–85. - PubMed
-
- Asahara T, Masuda H, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–8. - PubMed
-
- Asahara T, Murohara T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. - PubMed
-
- Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11(1):48–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

