Controlling silk fibroin particle features for drug delivery
- PMID: 20219241
- PMCID: PMC2846964
- DOI: 10.1016/j.biomaterials.2010.02.024
Controlling silk fibroin particle features for drug delivery
Abstract
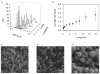
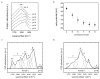
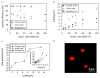
Silk proteins are a promising material for drug delivery due to their aqueous processability, biocompatibility, and biodegradability. A simple aqueous preparation method for silk fibroin particles with controllable size, secondary structure and zeta potential is reported. The particles were produced by salting out a silk fibroin solution with potassium phosphate. The effect of ionic strength and pH of potassium phosphate solution on the yield and morphology of the particles was determined. Secondary structure and zeta potential of the silk particles could be controlled by pH. Particles produced by salting out with 1.25 m potassium phosphate pH 6 showed a dominating silk II (crystalline) structure whereas particles produced at pH 9 were mainly composed of silk I (less crystalline). The results show that silk I-rich particles possess chemical and physical stability and secondary structure which remained unchanged during post treatments even upon exposure to 100% ethanol or methanol. A model is presented to explain the process of particle formation based on intra- and intermolecular interactions of the silk domains, influenced by pH and kosmotropic salts. The reported silk fibroin particles can be loaded with small molecule model drugs, such as alcian blue, rhodamine B, and crystal violet, by simple absorption based on electrostatic interactions. In vitro release of these compounds from the silk particles depends on charge-charge interactions between the compounds and the silk. With crystal violet we demonstrated that the release kinetics are dependent on the secondary structure of the particles.
Copyright (c) 2010 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Combinatorial effects of charge characteristics and hydrophobicity of silk fibroin on the sorption and release of charged dyes.J Biomater Sci Polym Ed. 2012;23(9):1199-215. doi: 10.1163/092050611X576972. Epub 2012 May 8. J Biomater Sci Polym Ed. 2012. PMID: 21639994
-
Structural insights into pH-responsive drug release of self-assembling human serum albumin-silk fibroin nanocapsules.Eur J Pharm Biopharm. 2018 Dec;133:176-187. doi: 10.1016/j.ejpb.2018.10.002. Epub 2018 Oct 3. Eur J Pharm Biopharm. 2018. PMID: 30291964
-
Preparation and characterization of silk fibroin as a biomaterial with potential for drug delivery.J Transl Med. 2012 Jun 7;10:117. doi: 10.1186/1479-5876-10-117. J Transl Med. 2012. PMID: 22676291 Free PMC article.
-
Preparation of silk fibroin carriers for controlled release.Microsc Res Tech. 2017 Mar;80(3):312-320. doi: 10.1002/jemt.22606. Epub 2015 Dec 6. Microsc Res Tech. 2017. PMID: 26638113 Review.
-
[Research Progress of Silk Fibroin As a Drug Delivery Materials].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2015 Dec;32(6):1364-8. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2015. PMID: 27079115 Review. Chinese.
Cited by
-
Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems.Pharmaceutics. 2023 Nov 14;15(11):2623. doi: 10.3390/pharmaceutics15112623. Pharmaceutics. 2023. PMID: 38004601 Free PMC article. Review.
-
Functionalising silk hydrogels with hetero- and homotypic nanoparticles.RSC Adv. 2024 Jan 22;14(5):3525-3535. doi: 10.1039/d3ra07634b. eCollection 2024 Jan 17. RSC Adv. 2024. PMID: 38259992 Free PMC article.
-
Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering.Int J Mol Sci. 2013 Jan 14;14(1):1629-54. doi: 10.3390/ijms14011629. Int J Mol Sci. 2013. PMID: 23344060 Free PMC article. Review.
-
Silk constructs for delivery of musculoskeletal therapeutics.Adv Drug Deliv Rev. 2012 Sep;64(12):1111-22. doi: 10.1016/j.addr.2012.03.016. Epub 2012 Apr 13. Adv Drug Deliv Rev. 2012. PMID: 22522139 Free PMC article. Review.
-
Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation.Biomaterials. 2020 Feb;232:119720. doi: 10.1016/j.biomaterials.2019.119720. Epub 2019 Dec 23. Biomaterials. 2020. PMID: 31896515 Free PMC article.
References
-
- Langer R. New methods of drug delivery. Science. 1990;249:1527–33. - PubMed
-
- Freiberg S, Zhu XX. Polymer microspheres for controlled drug release. Int J Pharm. 2004;282:1–18. - PubMed
-
- Edlund U, Albertsson AC. Degradable polymer microspheres for controlled drug delivery. In: Albertsson AC, editor. Degradable Aliphatic Polyesters. Berlin: Springer; 2002. pp. 67–112.
-
- Langer R, Peppas NA. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003;49:2990–3006.
-
- Daniel SK. Microparticles and nanoparticles for drug delivery. Biotechnol Bioeng. 2007;96:203–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

