Thermolability of mutant MMACHC protein in the vitamin B12-responsive cblC disorder
- PMID: 20219402
- PMCID: PMC2923755
- DOI: 10.1016/j.ymgme.2010.02.005
Thermolability of mutant MMACHC protein in the vitamin B12-responsive cblC disorder
Abstract
Methylmalonic aciduria and homocystinuria, cblC type, is the most common inborn error of cellular vitamin B12 metabolism. We previously showed that the protein carrying the mutation responsible for late-onset cblC (MMACHC-R161Q), treatable with high dose OHCbl, is able to bind OHCbl with wild-type affinity, leaving undetermined the disease mechanism involved [Froese et al., Mechanism of responsiveness, Mol. Genet. Metab. (2009).]. To assess whether the mutation renders the protein unstable, we investigated the thermostability of the wild-type and mutant MMACHC proteins, either unbound or bound to different cobalamins (Cbl), using differential scanning fluorimetry. We found that MMACHC-wt and MMACHC-R161Q are both very thermolabile proteins in their apo forms, with melting temperatures (T(m)) of 39.3+/-1.0 and 37.1+/-0.7 degrees C, respectively; a difference confirmed by unfolding of MMACHC-R161Q but not MMACHC-wt by isothermal denaturation at 35 degrees C over 120 min. However, with the addition of OHCbl, MMACHC-wt becomes significantly stabilized (Delta T(m max)=8 degrees C, half-maximal effective ligand concentration, AC(50)=3 microM). We surveyed the effect of different cobalamins on the stabilization of the wild-type protein and found that AdoCbl was the most stabilizing, exerting a maximum increase in T(m) of approximately 16 degrees C, followed by MeCbl at approximately 13 degrees C, each evaluated at 50 microM cofactor. The other cobalamins stabilized in the order (CN)(2)Cbi>OHCbl>CNCbl. Interestingly, the AC(50)'s for AdoCbl, MeCbl, (CN)(2)Cbi and OHCbl were similar and ranged from 1-3 microM, which compares well with the K(d) of 6 microM for OHCbl [Froese et al., Mechanism of responsiveness, Mol. Genet. Metab. (2009).]. Unlike MMACHC-wt, the mutant protein MMACHC-R161Q is only moderately stabilized by OHCbl (Delta T(m max)=4 degrees C). The dose-response curve also shows a lower effectivity of OHCbl with respect to stabilization, with an AC(50) of 7 microM. MMACHC-R161Q showed the same order of stabilization as MMACHC-wt, but each cobalamin stabilized this mutant protein less than its wild-type counterpart. Additionally, MMACHC-R161Q had a higher AC(50) for each cobalamin form compared to MMACHC-wt. Finally, we show that MMACHC-R161Q is able to support the base-off transition for AdoCbl and CNCbl, indicating this mutant is not blocked in that respect. Taken together, our results suggest that protein stability, as well as propensity for ligand-induced stabilization, contributes to the disease mechanism in late-onset cblC disorder. Our results underscore the importance of cofactor stabilization of MMACHC and suggest that even small increases in the concentration of cobalamin complexed with MMACHC may have therapeutic benefit in children with the late-onset, vitamin responsive cblC disease.
(c) 2010 Elsevier Inc. All rights reserved.
Figures

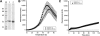
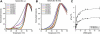
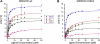

Similar articles
-
Mechanism of vitamin B12-responsiveness in cblC methylmalonic aciduria with homocystinuria.Mol Genet Metab. 2009 Dec;98(4):338-43. doi: 10.1016/j.ymgme.2009.07.014. Epub 2009 Aug 3. Mol Genet Metab. 2009. PMID: 19700356
-
Missense mutations in MMACHC protein from cblC disease affect its conformational stability and vitamin B12-binding activity: The example of R161Q mutation.Mol Genet Metab. 2025 Jul;145(3):109150. doi: 10.1016/j.ymgme.2025.109150. Epub 2025 May 22. Mol Genet Metab. 2025. PMID: 40441036
-
Modulation of conformational features and oligomerization of MMACHC by cobalamin variants: impact of the R161Q mutation in cblC disease.Eur Biophys J. 2025 Jun 27. doi: 10.1007/s00249-025-01777-5. Online ahead of print. Eur Biophys J. 2025. PMID: 40576712
-
Intracellular processing of vitamin B12 by MMACHC (CblC).Vitam Horm. 2022;119:275-298. doi: 10.1016/bs.vh.2022.02.001. Epub 2022 Mar 15. Vitam Horm. 2022. PMID: 35337623 Review.
-
Adult-onset CblC deficiency: a challenging diagnosis involving different adult clinical specialists.Orphanet J Rare Dis. 2022 Feb 2;17(1):33. doi: 10.1186/s13023-022-02179-y. Orphanet J Rare Dis. 2022. PMID: 35109910 Free PMC article. Review.
Cited by
-
Milder clinical and biochemical phenotypes associated with the c.482G>A (p.Arg161Gln) pathogenic variant in cobalamin C disease: Implications for management and screening.Mol Genet Metab. 2017 Sep;122(1-2):60-66. doi: 10.1016/j.ymgme.2017.06.011. Epub 2017 Jun 29. Mol Genet Metab. 2017. PMID: 28693988 Free PMC article.
-
Versatile enzymology and heterogeneous phenotypes in cobalamin complementation type C disease.iScience. 2022 Aug 18;25(9):104981. doi: 10.1016/j.isci.2022.104981. eCollection 2022 Sep 16. iScience. 2022. PMID: 36105582 Free PMC article. Review.
-
Interaction of Glutathione with MMACHC Arginine-Rich Pocket Variants Associated with Cobalamin C Disease: Insights from Molecular Modeling.Biomedicines. 2023 Dec 4;11(12):3217. doi: 10.3390/biomedicines11123217. Biomedicines. 2023. PMID: 38137438 Free PMC article.
-
The unusual substrate specificity of a virulence associated serine hydrolase from the highly toxic bacterium, Francisella tularensis.Biochem Biophys Rep. 2016 Jul 12;7:415-422. doi: 10.1016/j.bbrep.2016.07.006. eCollection 2016 Sep. Biochem Biophys Rep. 2016. PMID: 28955933 Free PMC article.
-
Inter-domain communication of human cystathionine β-synthase: structural basis of S-adenosyl-L-methionine activation.J Biol Chem. 2014 Dec 26;289(52):36018-30. doi: 10.1074/jbc.M114.610782. Epub 2014 Oct 21. J Biol Chem. 2014. PMID: 25336647 Free PMC article.
References
-
- Hodgkin D.C., Kamper J., Mackay M., Pickworth J., Trueblood K.N., White J.G. Structure of vitamin B12. Nature. 1956;178:64–66. - PubMed
-
- Smith E.L. Purification of anti-pernicious anaemia factors from liver. Nature. 1948;161:638. - PubMed
-
- Rickes E.L., Brink N.G., Koniuszy F.R., Wood T.R., Folkers K. Crystalline vitamin B12. Science. 1948;107:396–397. - PubMed
-
- Qureshi A.A., Rosenblatt D.S., Cooper B.A. Inherited disorders of cobalamin metabolism. Crit. Rev. Oncol. Hematol. 1994;17:133–151. - PubMed
-
- Rosenblatt D.S., Fenton W.A. Inherited disorders of folate and cobalamin transport and metabolism. In: Scriver C.R., Beaudet A.L., Valle D., Sly W.S., Childs B., Kinzler K.W., Vogelstein B., editors. The Metabolic and Molecular Bases of Inherited Diseases. McGraw-Hill; New York: 2001. pp. 3897–3933.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

