Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium
- PMID: 20219464
- PMCID: PMC4828244
- DOI: 10.1016/j.exppara.2010.02.009
Cysteamine, the natural metabolite of pantetheinase, shows specific activity against Plasmodium
Abstract
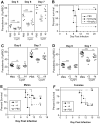
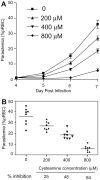
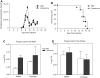
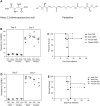
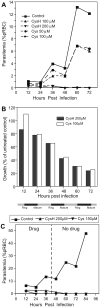
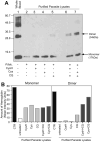
In mice, loss of pantetheinase activity causes susceptibility to infection with Plasmodium chabaudi AS. Treatment of mice with the pantetheinase metabolite cysteamine reduces blood-stage replication of P. chabaudi and significantly increases survival. Similarly, a short exposure of Plasmodium to cysteamine ex vivo is sufficient to suppress parasite infectivity in vivo. This effect of cysteamine is specific and not observed with a related thiol (dimercaptosuccinic acid) or with the pantethine precursor of cysteamine. Also, cysteamine does not protect against infection with the parasite Trypanosoma cruzi or the fungal pathogen Candida albicans, suggesting cysteamine acts directly against the parasite and does not modulate host inflammatory response. Cysteamine exposure also blocks replication of P. falciparum in vitro; moreover, these treated parasites show higher levels of intact hemoglobin. This study highlights the in vivo action of cysteamine against Plasmodium and provides further evidence for the involvement of pantetheinase in host response to this infection.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Cysteamine broadly improves the anti-plasmodial activity of artemisinins against murine blood stage and cerebral malaria.Malar J. 2016 May 6;15(1):260. doi: 10.1186/s12936-016-1317-3. Malar J. 2016. PMID: 27150250 Free PMC article.
-
Cysteamine, the molecule used to treat cystinosis, potentiates the antimalarial efficacy of artemisinin.Antimicrob Agents Chemother. 2010 Aug;54(8):3262-70. doi: 10.1128/AAC.01719-09. Epub 2010 May 17. Antimicrob Agents Chemother. 2010. PMID: 20479197 Free PMC article.
-
Violacein extracted from Chromobacterium violaceum inhibits Plasmodium growth in vitro and in vivo.Antimicrob Agents Chemother. 2009 May;53(5):2149-52. doi: 10.1128/AAC.00693-08. Epub 2009 Mar 9. Antimicrob Agents Chemother. 2009. PMID: 19273690 Free PMC article.
-
Genetic analysis in mice identifies cysteamine as a novel partner for artemisinin in the treatment of malaria.Mamm Genome. 2011 Aug;22(7-8):486-94. doi: 10.1007/s00335-011-9316-8. Epub 2011 Mar 25. Mamm Genome. 2011. PMID: 21437649 Review.
-
Fitness of drug-resistant malaria parasites.Acta Trop. 2005 Jun;94(3):251-9. doi: 10.1016/j.actatropica.2005.04.005. Epub 2005 Apr 18. Acta Trop. 2005. PMID: 15845348 Review.
Cited by
-
Cysteamine broadly improves the anti-plasmodial activity of artemisinins against murine blood stage and cerebral malaria.Malar J. 2016 May 6;15(1):260. doi: 10.1186/s12936-016-1317-3. Malar J. 2016. PMID: 27150250 Free PMC article.
-
The Pathophysiological Role of CoA.Int J Mol Sci. 2020 Nov 28;21(23):9057. doi: 10.3390/ijms21239057. Int J Mol Sci. 2020. PMID: 33260564 Free PMC article. Review.
-
Host genetics in malaria: lessons from mouse studies.Mamm Genome. 2018 Aug;29(7-8):507-522. doi: 10.1007/s00335-018-9744-9. Epub 2018 Mar 28. Mamm Genome. 2018. PMID: 29594458 Review.
-
Genetic control of susceptibility to infection with Plasmodium chabaudi chabaudi AS in inbred mouse strains.Genes Immun. 2012 Feb;13(2):155-63. doi: 10.1038/gene.2011.67. Epub 2011 Oct 6. Genes Immun. 2012. PMID: 21975430 Free PMC article.
-
Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides.PLoS One. 2014 Mar 19;9(3):e91686. doi: 10.1371/journal.pone.0091686. eCollection 2014. PLoS One. 2014. PMID: 24646894 Free PMC article.
References
-
- Aitman TJ, Cooper LD, Norsworthy PJ, Wahid FN, Gray JK, Curtis BR, McKeigue PM, Kwiatkowski D, Greenwood BM, Snow RW, Hill AV, Scott J. Malaria susceptibility and CD36 mutation. Nature. 2000;405:1015–1016. - PubMed
-
- Anikster Y, Shotelersuk V, Gahl WA. CTNS mutations in patients with cystinosis. Human Mutation. 1999;14:454–458. - PubMed
-
- Aposhian HV. DMSA and DMPS–water soluble antidotes for heavy metal poisoning. Annual Reviews of Pharmacology and Toxicology. 1983;23:193–215. - PubMed
-
- Ayi K, Min-Oo G, Serghides L, Crockett M, Kirby-Allen M, Quirt I, Gros P, Kain KC. Pyruvate kinase deficiency and malaria. New England Journal of Medicine. 2008;358:1805–1810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

