Hypertension in autosomal dominant polycystic kidney disease
- PMID: 20219618
- PMCID: PMC2845913
- DOI: 10.1053/j.ackd.2010.01.001
Hypertension in autosomal dominant polycystic kidney disease
Abstract
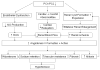
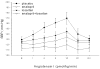
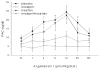
Hypertension is common and occurs in a majority of autosomal dominant polycystic kidney disease (ADPKD) patients before the loss of kidney function. Hypertension relates to progressive kidney enlargement and is a significant independent risk factor for progression to ESRD. The pathogenesis of hypertension in ADPKD is complex and dependent on many factors that influence each other. Pkd1 and Pkd2 expression levels are highest in the major vessels and are present in the cilia of endothelial cells and in vascular smooth muscle cells. Decreased or absent polycystin 1 or 2 expression is associated with abnormal vascular structure and function. Pkd1/Pkd2 deficiency results in reduced nitric oxide (NO) levels, altered endothelial response to shear stress with attenuation in vascular relaxation. Ten percent to 20% of ADPKD children show hypertension and the majority of adults are hypertensive before any loss of kidney function. Cardiac abnormalities such as left ventricular hypertrophy and carotid intimal wall thickening are present before the development of hypertension in ADPKD. The activation of the renin-angiotensin-aldosterone system occurs in ADPKD because of decreased NO production as well as bilateral cyst expansion and intrarenal ischemia. With increasing cyst size, further activation of the RAAS occurs, blood pressure increases, and a vicious cycle ensues with enhanced cyst growth and hypertension ultimately leading to ESRD. The inhibition of the angiotensin aldosterone system is possible with angiotensin converting enzyme inhibitors and angiotensin receptor blockers. However, interventional studies have not yet shown benefit in slowing progression to renal failure in ADPKD. Currently, large multicenter studies are being performed to determine the beneficial effects of RAAS inhibition both early and late in ADPKD.
2010 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. - PubMed
-
- Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol. 2008;3:1197–1204. - PubMed
-
- Perrone RD, Ruthazer R, Terrin NC. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am J Kidney Dis. 2001;38:777–784. - PubMed
-
- Rahman E, Niaz FA, Al-Suwaida A, et al. Analysis of causes of mortality in patients with autosomal dominant polycystic kidney disease: a single center study. Saudi J Kidney Dis Transpl. 2009;20:806–810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

