Aluminium reduces sugar uptake in tobacco cell cultures: a potential cause of inhibited elongation but not of toxicity
- PMID: 20219776
- PMCID: PMC2852655
- DOI: 10.1093/jxb/erq027
Aluminium reduces sugar uptake in tobacco cell cultures: a potential cause of inhibited elongation but not of toxicity
Abstract
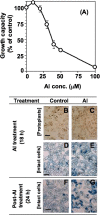
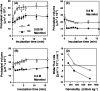
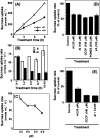
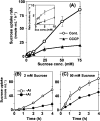
Aluminium is well known to inhibit plant elongation, but the role in this inhibition played by water relations remains unclear. To investigate this, tobacco (Nicotiana tabacum L.) suspension-cultured cells (line SL) was used, treating them with aluminium (50 microM) in a medium containing calcium, sucrose, and MES (pH 5.0). Over an 18 h treatment period, aluminium inhibited the increase in fresh weight almost completely and decreased cellular osmolality and internal soluble sugar content substantially; however, aluminium did not affect the concentrations of major inorganic ions. In aluminium-treated cultures, fresh weight, soluble sugar content, and osmolality decreased over the first 6 h and remained constant thereafter, contrasting with their continued increases in the untreated cultures. The rate of sucrose uptake, measured by radio-tracer, was reduced by approximately 60% within 3 h of treatment. Aluminium also inhibited glucose uptake. In an aluminium-tolerant cell line (ALT301) isogenic to SL, all of the above-mentioned changes in water relations occurred and tolerance emerged only after 6 h and appeared to involve the suppression of reactive oxygen species. Further separating the effects of aluminium on elongation and cell survival, sucrose starvation for 18 h inhibited elongation and caused similar changes in cellular osmolality but stimulated the production of neither reactive oxygen species nor callose and did not cause cell death. We propose that the inhibition of sucrose uptake is a mechanism whereby aluminium inhibits elongation, but does not account for the induction of cell death.
Figures





Similar articles
-
An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells.J Inorg Biochem. 2003 Sep 15;97(1):59-68. doi: 10.1016/s0162-0134(03)00182-x. J Inorg Biochem. 2003. PMID: 14507461
-
Mitochondrial alterations related to programmed cell death in tobacco cells under aluminium stress.C R Biol. 2008 Aug;331(8):597-610. doi: 10.1016/j.crvi.2008.04.008. Epub 2008 Jun 2. C R Biol. 2008. PMID: 18606389
-
Aluminium-induced cell death requires upregulation of NtVPE1 gene coding vacuolar processing enzyme in tobacco (Nicotiana tabacum L.).J Inorg Biochem. 2018 Apr;181:152-161. doi: 10.1016/j.jinorgbio.2017.09.008. Epub 2017 Sep 12. J Inorg Biochem. 2018. PMID: 28967473
-
Degradation of membrane phospholipids in plant cells cultured in sucrose-free medium.Autophagy. 2006 Jul-Sep;2(3):244-6. doi: 10.4161/auto.2745. Epub 2006 Jul 29. Autophagy. 2006. PMID: 16874091 Review.
-
Role of magnesium in alleviation of aluminium toxicity in plants.J Exp Bot. 2011 Apr;62(7):2251-64. doi: 10.1093/jxb/erq456. Epub 2011 Jan 27. J Exp Bot. 2011. PMID: 21273333 Review.
Cited by
-
External aluminium supply regulates photosynthesis and carbon partitioning in the Al-accumulating tropical shrub Melastoma malabathricum.PLoS One. 2024 Mar 20;19(3):e0297686. doi: 10.1371/journal.pone.0297686. eCollection 2024. PLoS One. 2024. PMID: 38507439 Free PMC article.
-
Oxygen supply is a prerequisite for response to aluminum in cultured cells of tobacco (Nicotiana tabacum).Plant Cell Physiol. 2025 Aug 12;66(7):1044-1060. doi: 10.1093/pcp/pcaf055. Plant Cell Physiol. 2025. PMID: 40421753 Free PMC article.
-
Phytotoxic and genotoxic effect of Aluminum to date palm (Phoenix dactylifera L.) in vitro cultures.J Genet Eng Biotechnol. 2019 Oct 21;17(1):7. doi: 10.1186/s43141-019-0007-2. J Genet Eng Biotechnol. 2019. PMID: 31659544 Free PMC article.
-
Formation of Proto-Kranz in C3 Rice Induced by Spike-Stalk Injection Method.Int J Mol Sci. 2021 Apr 21;22(9):4305. doi: 10.3390/ijms22094305. Int J Mol Sci. 2021. Retraction in: Int J Mol Sci. 2021 Nov 03;22(21):11930. doi: 10.3390/ijms222111930. PMID: 33919137 Free PMC article. Retracted.
-
Integrated transcriptomics and metabolomics provides insights into the Nicotiana tabacum response to heat stress.Front Plant Sci. 2024 Jul 22;15:1425944. doi: 10.3389/fpls.2024.1425944. eCollection 2024. Front Plant Sci. 2024. PMID: 39109058 Free PMC article.
References
-
- Ahn SJ, Sivaguru M, Chung GC, Rengel Z, Matsumoto H. Aluminium-induced growth inhibition is associated with impaired efflux and influx of H+across the plasma membrane in root apices of squash (Cucurbita pepo) Journal of Experimental Botany. 2002;53:1959–1966. - PubMed
-
- Chaen H, Nakada T, Mukai N, Nishimoto T, Fukuda S, Sugimoto T, Kurimoto M, Tsujisaka Y. Efficient enzymatic synthesis of disaccharide, α-d-glactosyl α-d-glucoside, by trehalose phosphorylase from Thermoanaerobacter brockii. Journal of Applied Glycoscience. 2001;48:135–137.
-
- Chang Y-C, Yamamoto Y, Matsumoto H. Enhancement of callose production by a combination of aluminum and iron in suspension-cultured tobacco cells. Soil Science and Plant Nutrition. 1999;45:337–347.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

