Removing the mustard oil bomb from seeds: transgenic ablation of myrosin cells in oilseed rape (Brassica napus) produces MINELESS seeds
- PMID: 20219777
- PMCID: PMC2852662
- DOI: 10.1093/jxb/erq039
Removing the mustard oil bomb from seeds: transgenic ablation of myrosin cells in oilseed rape (Brassica napus) produces MINELESS seeds
Abstract
Many plant phytochemicals constitute binary enzyme-glucoside systems and function in plant defence. In brassicas, the enzyme myrosinase is confined to specific myrosin cells that separate the enzyme from its substrate; the glucosinolates. The myrosinase-catalysed release of toxic and bioactive compounds such as isothiocyanates, upon activation or tissue damage, has been termed 'the mustard oil bomb' and characterized as a 'toxic mine' in plant defence. The removal of myrosin cells and the enzyme that triggers the release of phytochemicals have been investigated by genetically modifying Brassica napus plants to remove myrosinase-storing idioblasts. A construct with the seed myrosin cell-specific Myr1.Bn1 promoter was used to express a ribonuclease, barnase. Transgenic plants ectopically expressing barnase were embryo lethal. Co-expressing barnase under the control of the Myr1.Bn1 promoter with the barnase inhibitor, barstar, under the control of the cauliflower mosaic virus 35S promoter enabled a selective and controlled death of myrosin cells without affecting plant viability. Ablation of myrosin cells was confirmed with light and electron microscopy, with immunohistological analysis and immunogold-electron microscopy analysis showing empty holes where myrosin cells normally are localized. Further evidence for a successful myrosin cell ablation comes from immunoblots showing absence of myrosinase and negligible myrosinase activity, and autolysis experiments showing negligible production of glucosinolate hydrolysis products. The plants where the myrosin defence cells have been ablated and named 'MINELESS plants'. The epithiospecifier protein profile and glucosinolate levels were changed in MINELESS plants, pointing to localization of myrosinases and a 35 kDa epithiospecifier protein in myrosin cells and a reduced turnover of glucosinolates in MINELESS plants.
Figures




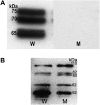



References
-
- Ahuja I, Rohloff J, Bones AM. Defence mechanisms of Brassicaceae: implications for plant–insect interactions and potential for integrated pest management. A review. Agronomy for Sustainable Development. 2009 DOI: 10.1051/agro/2009025.
-
- Barth C, Jander G. Arabidopsis myrosinases TGG1 and TGG2 have redundant function in glucosinolate breakdown and insect defense. The Plant Journal. 2006;46:549–562. - PubMed
-
- Bednarek P, Pislewska-Bednarek M, et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

