Omi/HtrA2 protease is associated with tubular cell apoptosis and fibrosis induced by unilateral ureteral obstruction
- PMID: 20219823
- PMCID: PMC2886814
- DOI: 10.1152/ajprenal.00737.2009
Omi/HtrA2 protease is associated with tubular cell apoptosis and fibrosis induced by unilateral ureteral obstruction
Abstract
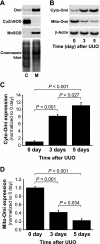
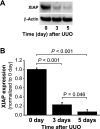
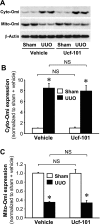
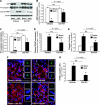
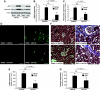
Kidney fibrosis, a typical characteristic of chronic renal disease, is associated with tubular epithelial cell apoptosis. The results of our recent studies have shown that Omi/HtrA2 (Omi), a proapoptotic mitochondrial serine protease, performs a crucial function in renal tubular epithelial apoptotic cell death in animal models of acute kidney injury, including cisplatin toxicity and ischemia-reperfusion insult. However, the role of Omi in tubulointerstitial disease-associated fibrosis in the kidney remains to be clearly defined. We evaluated the potential function and molecular mechanism of Omi in ureteral obstruction-induced kidney epithelial cell apoptosis and fibrosis. The mice were subjected to unilateral ureteral obstruction (UUO) via the ligation of the left ureter near the renal pelvis. UUO increased the protein level of Omi in the cytosolic fraction of the kidney, with a concomitant reduction in the mitochondrial fraction. UUO reduced the X-linked inhibitor of apoptosis protein (XIAP), a substrate of Omi, and pro-caspase-3, whereas it increased cleaved poly(ADP-ribose) polymerase (cleaved PARP) and the number of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive cells. When mice were treated with ucf-101, an inhibitor of the proteolytic activity of Omi (6.19 microg/day ip), on a daily basis beginning 2 days before UUO and continuing until the end of the experiment, the Omi inhibitor protected XIAP cleavage after UUO and reduced the increment of PARP cleavage and the numbers of TUNEL-positive cells. Furthermore, the Omi inhibitor significantly attenuated UUO-induced increases in fibrotic characteristics in the kidney, including the atrophy and dilation of tubules, expansion of the interstitium, and increases in the expression of collagens, alpha-smooth muscle actin, and fibronectin. In conclusion, Omi/HtrA2 is associated with apoptotic signaling pathways in tubular epithelial cells activated by unilateral ureteral obstruction, thereby resulting in kidney fibrosis.
Figures


Similar articles
-
Omi/HtrA2 Regulates a Mitochondria-Dependent Apoptotic Pathway in a Murine Model of Septic Encephalopathy.Cell Physiol Biochem. 2018;49(6):2163-2173. doi: 10.1159/000493819. Epub 2018 Oct 4. Cell Physiol Biochem. 2018. PMID: 30286467
-
Enhanced HtrA2/Omi expression in oxidative injury to retinal pigment epithelial cells and murine models of neurodegeneration.Invest Ophthalmol Vis Sci. 2009 Oct;50(10):4957-66. doi: 10.1167/iovs.09-3381. Epub 2009 May 14. Invest Ophthalmol Vis Sci. 2009. PMID: 19443712 Free PMC article.
-
Ulinastatin inhibits renal tubular epithelial apoptosis and interstitial fibrosis in rats with unilateral ureteral obstruction.Mol Med Rep. 2017 Dec;16(6):8916-8922. doi: 10.3892/mmr.2017.7692. Epub 2017 Oct 3. Mol Med Rep. 2017. PMID: 28990075 Free PMC article.
-
Obstructive nephropathy: lessons from cystic kidney disease.Nephron. 2000 Jan;84(1):6-12. doi: 10.1159/000045532. Nephron. 2000. PMID: 10644902 Review.
-
Renal fibrosis progression following partial unilateral ureteral obstruction: mechanisms and therapeutic insights.World J Urol. 2025 Apr 17;43(1):229. doi: 10.1007/s00345-025-05580-x. World J Urol. 2025. PMID: 40244436 Free PMC article. Review.
Cited by
-
Cell biology of ischemia/reperfusion injury.Int Rev Cell Mol Biol. 2012;298:229-317. doi: 10.1016/B978-0-12-394309-5.00006-7. Int Rev Cell Mol Biol. 2012. PMID: 22878108 Free PMC article. Review.
-
Apoptosis and acute kidney injury.Kidney Int. 2011 Jul;80(1):29-40. doi: 10.1038/ki.2011.120. Epub 2011 May 11. Kidney Int. 2011. PMID: 21562469 Free PMC article. Review.
-
Mitochondrial Unfolded Protein Response (mtUPR) and Diseases.Curr Med Chem. 2025;32(9):1674-1684. doi: 10.2174/0929867331666230822095924. Curr Med Chem. 2025. PMID: 37608662 Review.
-
Ischemia/Reperfusion.Compr Physiol. 2016 Dec 6;7(1):113-170. doi: 10.1002/cphy.c160006. Compr Physiol. 2016. PMID: 28135002 Free PMC article. Review.
-
Protective Effects of UCF-101 on Cerebral Ischemia-Reperfusion (CIR) is Depended on the MAPK/p38/ERK Signaling Pathway.Cell Mol Neurobiol. 2016 Aug;36(6):907-914. doi: 10.1007/s10571-015-0275-6. Epub 2015 Oct 1. Cell Mol Neurobiol. 2016. PMID: 26429193 Free PMC article.
References
-
- Althaus J, Siegelin MD, Dehghani F, Cilenti L, Zervos AS, Rami A. The serine protease Omi/HtrA2 is involved in XIAP cleavage and in neuronal cell death following focal cerebral ischemia/reperfusion. Neurochem Int 50: 172–180, 2007 - PubMed
-
- Bhuiyan MS, Fukunaga K. Activation of HtrA2, a mitochondrial serine protease mediates apoptosis: current knowledge on HtrA2 mediated myocardial ischemia/reperfusion injury. Cardiovasc Ther 26: 224–232, 2008 - PubMed
-
- Bhuiyan MS, Fukunaga K. Inhibition of HtrA2/Omi ameliorates heart dysfunction following ischemia/reperfusion injury in rat heart in vivo. Eur J Pharmacol 557: 168–177, 2007 - PubMed
-
- Cilenti L, Kyriazis GA, Soundarapandian MM, Stratico V, Yerkes A, Park KM, Sheridan AM, Alnemri ES, Bonventre JV, Zervos AS. Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am J Physiol Renal Physiol 288: F371–F379, 2005 - PubMed
-
- Cilenti L, Lee Y, Hess S, Srinivasula S, Park KM, Junqueira D, Davis H, Bonventre JV, Alnemri ES, Zervos AS. Characterization of a novel and specific inhibitor for the pro-apoptotic protease Omi/HtrA2. J Biol Chem 278: 11489–11494, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

