Cancer cell-specific internalizing ligands from phage displayed beta-lactamase-peptide fusion libraries
- PMID: 20219829
- PMCID: PMC2865360
- DOI: 10.1093/protein/gzq013
Cancer cell-specific internalizing ligands from phage displayed beta-lactamase-peptide fusion libraries
Abstract
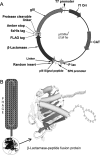
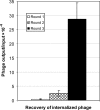
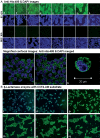
The present study was focused on identifying cancer cell-specific internalizing ligands using a new kind of phage display library in which the linear or cysteine-constrained random peptides were at amino-terminus fusion to catalytically active P99 beta-lactamase molecules. The size and quality of libraries were comparable to other reported phage display systems. Several cancer cell-specific binding and internalizing beta-lactamase-peptide fusion ligands were isolated by selecting these libraries on the live BT-474 human breast cancer cells. The identified ligands shared several significant motifs, which showed their selectivity and possible binding to some common cancer cell targets. The beta-lactamase fusion made the whole process of clone screening efficient and simple. The ligands selected from such libraries do not require peptide synthesis and modifications, and can be used directly for applications that require ligand tracking. In addition, the selected beta-lactamase-peptide ligands have a potential for their direct use in targeted enzyme prodrug therapy. The cancer-specific peptides can also be adopted for other kinds of targeted delivery protocols requiring cell-specific affinity reagents. This is first report on the selection of cell-internalized enzyme conjugates using phage display technology, which opens the possibility for new fusion libraries with other relevant enzymes.
Figures
Similar articles
-
Phage-displayed combinatorial peptide libraries in fusion to beta-lactamase as reporter for an accelerated clone screening: Potential uses of selected enzyme-linked affinity reagents in downstream applications.Comb Chem High Throughput Screen. 2010 Jan;13(1):75-87. doi: 10.2174/138620710790218258. Comb Chem High Throughput Screen. 2010. PMID: 20214576 Free PMC article.
-
Novel beta-lactamase-random peptide fusion libraries for phage display selection of cancer cell-targeting agents suitable for enzyme prodrug therapy.J Drug Target. 2010 Feb;18(2):115-24. doi: 10.3109/10611860903244181. J Drug Target. 2010. PMID: 19751096 Free PMC article.
-
Towards a ligand targeted enzyme prodrug therapy: single round panning of a beta-lactamase scaffold library on human cancer cells.Int J Cancer. 2007 May 15;120(10):2233-42. doi: 10.1002/ijc.22138. Int J Cancer. 2007. PMID: 17285581
-
Designing scaffolds of peptides for phage display libraries.J Biosci Bioeng. 2005 May;99(5):448-56. doi: 10.1263/jbb.99.448. J Biosci Bioeng. 2005. PMID: 16233816 Review.
-
Screening peptide array library for the identification of cancer cell-binding peptides.Methods Mol Biol. 2015;1248:239-47. doi: 10.1007/978-1-4939-2020-4_16. Methods Mol Biol. 2015. PMID: 25616337 Review.
Cited by
-
Landscape phages and their fusion proteins targeted to breast cancer cells.Protein Eng Des Sel. 2012 Jun;25(6):271-83. doi: 10.1093/protein/gzs013. Epub 2012 Apr 6. Protein Eng Des Sel. 2012. PMID: 22490956 Free PMC article.
-
Development of a new affinity maturation protocol for the construction of an internalizing anti-nucleolin antibody library.Sci Rep. 2024 May 8;14(1):10608. doi: 10.1038/s41598-024-61230-z. Sci Rep. 2024. PMID: 38719911 Free PMC article.
-
In Vitro Selection of Cancer Cell-Specific Molecular Recognition Elements from Amino Acid Libraries.J Immunol Res. 2015;2015:186586. doi: 10.1155/2015/186586. Epub 2015 Sep 7. J Immunol Res. 2015. PMID: 26436100 Free PMC article. Review.
-
Combinatorial peptide libraries: mining for cell-binding peptides.Chem Rev. 2014 Jan 22;114(2):1020-81. doi: 10.1021/cr400166n. Epub 2013 Dec 3. Chem Rev. 2014. PMID: 24299061 Free PMC article. Review. No abstract available.
-
Intravenous infusion of phage-displayed antibody library in human cancer patients: enrichment and cancer-specificity of tumor-homing phage-antibodies.Cancer Immunol Immunother. 2013 Aug;62(8):1397-410. doi: 10.1007/s00262-013-1443-5. Epub 2013 Jun 5. Cancer Immunol Immunother. 2013. PMID: 23736951 Free PMC article. Clinical Trial.
References
-
- Allen T.M. Nat. Rev. 2002;2:750–763. - PubMed
-
- Bagshawe K.D. Curr. Drug Targets. 2009;10:152–157. - PubMed
-
- Becerril B., Poul M.A., Marks J.D. Biochem. Biophys. Res. Commun. 1999;255:386–393. - PubMed
-
- Begent R.H., et al. Nat. Med. 1996;2:979–984. - PubMed
-
- Brissette R., Goldstein N.I. Methods Mol. Biol. 2007;383:203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

