Regulatory interactions between muscle and the immune system during muscle regeneration
- PMID: 20219869
- PMCID: PMC2867520
- DOI: 10.1152/ajpregu.00735.2009
Regulatory interactions between muscle and the immune system during muscle regeneration
Abstract
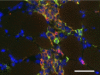
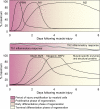
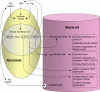
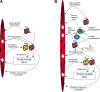
Recent discoveries reveal complex interactions between skeletal muscle and the immune system that regulate muscle regeneration. In this review, we evaluate evidence that indicates that the response of myeloid cells to muscle injury promotes muscle regeneration and growth. Acute perturbations of muscle activate a sequence of interactions between muscle and inflammatory cells. The initial inflammatory response is a characteristic Th1 inflammatory response, first dominated by neutrophils and subsequently by CD68(+) M1 macrophages. M1 macrophages can propagate the Th1 response by releasing proinflammatory cytokines and cause further tissue damage through the release of nitric oxide. Myeloid cells in the early Th1 response stimulate the proliferative phase of myogenesis through mechanisms mediated by TNF-alpha and IL-6; experimental prolongation of their presence is associated with delayed transition to the early differentiation stage of myogenesis. Subsequent invasion by CD163(+)/CD206(+) M2 macrophages attenuates M1 populations through the release of anti-inflammatory cytokines, including IL-10. M2 macrophages play a major role in promoting growth and regeneration; their absence greatly slows muscle growth following injury or modified use and inhibits muscle differentiation and regeneration. Chronic muscle injury leads to profiles of macrophage invasion and function that differ from acute injuries. For example, mdx muscular dystrophy yields invasion of muscle by M1 macrophages, but their early invasion is accompanied by a subpopulation of M2a macrophages. M2a macrophages are IL-4 receptor(+)/CD206(+) cells that reduce cytotoxicity of M1 macrophages. Subsequent invasion of dystrophic muscle by M2c macrophages is associated with progression of the regenerative phase in pathophysiology. Together, these findings show that transitions in macrophage phenotype are an essential component of muscle regeneration in vivo following acute or chronic muscle damage.
Figures

References
-
- Abood EA, Jones MM. Macrophages in developing mammalian skeletal muscle: evidence for muscle fibre death as a normal developmental event. Acta Anat (Basel) 140: 201–212, 1991 - PubMed
-
- Backman E, Henriksson KG. Low-dose prednisolone treatment in Duchenne and Becker muscular dystrophy. Neuromusc Disord 5: 233–241, 1995 - PubMed
-
- Bartoli C, Civatte M, Pellissier JF, Figarella-Branger D. CCR2A and CCR2B, the two isoforms of the monocyte chemoattractant protein-1 receptor are up-regulated and expressed by different cell subsets in idiopathic inflammatory myopathies. Acta Neuropathol (Berl) 102: 385–392, 2001 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

