Modulation of gene expression related to Toll-like receptor signaling in dendritic cells by poly(gamma-glutamic acid) nanoparticles
- PMID: 20219877
- PMCID: PMC2863380
- DOI: 10.1128/CVI.00505-09
Modulation of gene expression related to Toll-like receptor signaling in dendritic cells by poly(gamma-glutamic acid) nanoparticles
Abstract
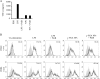
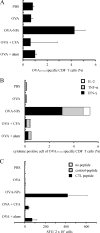
Poly(gamma-glutamic acid) (gamma-PGA) nanoparticles (NPs) have previously been reported as an efficient antigen delivery system with adjuvant activity. In this study, the gene expression in murine bone marrow-derived dendritic cells (DCs) treated with gamma-PGA NPs was examined by oligonucleotide microarray analysis and compared with that in cells treated with other adjuvants. The gene expression of proinflammatory chemokines, cytokines, and costimulatory molecules was upregulated considerably in DCs treated with gamma-PGA NPs. The upregulation pattern was similar to that in DCs treated with lipopolysaccharide (LPS) but not to that in DCs treated with unparticulate gamma-PGA. The activation of DCs by gamma-PGA NPs was confirmed by real-time reverse transcriptase PCR (RT-PCR) analysis of genes related to Toll-like receptor (TLR) signaling. The effect of gamma-PGA NPs on DCs was not annihilated by treatment with polymyxin B, an inhibitor of LPS. Furthermore, the immunization of mice with gamma-PGA NPs carrying ovalbumin (OVA) as an antigen significantly induced antigen-specific CD8(+) T cells and antigen-specific production of interleukin-2, tumor necrosis factor alpha, and gamma interferon from the cells. Such activities of gamma-PGA NPs were more potent than those obtained with immunization with OVA plus aluminum hydroxide or OVA plus complete Freund's adjuvant. These results suggest that gamma-PGA NPs induce a CD8(+) T-cell response by activating innate immunity in a fashion different from that of LPS. Thus, gamma-PGA NPs may be an attractive candidate to be developed further as a vaccine adjuvant.
Figures


Similar articles
-
Modulation of innate and adaptive immunity by biodegradable nanoparticles.Immunol Lett. 2009 Jun 30;125(1):46-52. doi: 10.1016/j.imlet.2009.05.008. Epub 2009 Jun 6. Immunol Lett. 2009. PMID: 19505507
-
Comparative activity of biodegradable nanoparticles with aluminum adjuvants: antigen uptake by dendritic cells and induction of immune response in mice.Immunol Lett. 2011 Oct 30;140(1-2):36-43. doi: 10.1016/j.imlet.2011.06.002. Epub 2011 Jun 12. Immunol Lett. 2011. PMID: 21693134
-
Poly(gamma-glutamic acid) nanoparticles as an efficient antigen delivery and adjuvant system: potential for an AIDS vaccine.J Med Virol. 2008 Jan;80(1):11-9. doi: 10.1002/jmv.21029. J Med Virol. 2008. PMID: 18041033
-
Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant.Adv Drug Deliv Rev. 2013 Oct;65(10):1386-99. doi: 10.1016/j.addr.2013.05.013. Epub 2013 Jun 7. Adv Drug Deliv Rev. 2013. PMID: 23751781 Review.
-
[Efficacy and safety of poly (gamma-glutamic acid) based nanoparticles (gamma-PGA NPs) as vaccine carrier].Yakugaku Zasshi. 2008 Nov;128(11):1559-65. doi: 10.1248/yakushi.128.1559. Yakugaku Zasshi. 2008. PMID: 18981690 Review. Japanese.
Cited by
-
Tolerogenic Transcriptional Signatures of Steady-State and Pathogen-Induced Dendritic Cells.Front Immunol. 2018 Feb 28;9:333. doi: 10.3389/fimmu.2018.00333. eCollection 2018. Front Immunol. 2018. PMID: 29541071 Free PMC article. Review.
-
Nanovectorized radiotherapy: a new strategy to induce anti-tumor immunity.Front Oncol. 2012 Oct 10;2:136. doi: 10.3389/fonc.2012.00136. eCollection 2012. Front Oncol. 2012. PMID: 23087900 Free PMC article.
-
Metallic Nanoparticles: Their Potential Role in Breast Cancer Immunotherapy via Trained Immunity Provocation.Biomedicines. 2023 Apr 23;11(5):1245. doi: 10.3390/biomedicines11051245. Biomedicines. 2023. PMID: 37238916 Free PMC article. Review.
-
The use of nanolipoprotein particles to enhance the immunostimulatory properties of innate immune agonists against lethal influenza challenge.Biomaterials. 2013 Dec;34(38):10305-18. doi: 10.1016/j.biomaterials.2013.09.038. Epub 2013 Sep 27. Biomaterials. 2013. PMID: 24075406 Free PMC article.
-
In Vivo Modulation of Dendritic Cells by Engineered Materials: Towards New Cancer Vaccines.Nano Today. 2011 Oct;6(5):466-477. doi: 10.1016/j.nantod.2011.08.005. Nano Today. 2011. PMID: 22125572 Free PMC article.
References
-
- Akagi, T., T. Kaneko, T. Kida, and M. Akashi. 2005. Preparation and characterization of biodegradable nanoparticles based on poly(gamma-glutamic acid) with l-phenylalanine as a protein carrier. J. Control. Release 108:226-236. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
-
- Chiew Tong, N. K., J. Beran, S. A. Kee, J. L. Miguel, C. Sanchez, J. M. Bayas, A. Vilella, J. R. de Juanes, P. Arrazola, F. Calbo-Torrecillas, E. L. de Novales, V. Hamtiaux, M. Lievens, and M. Stoffel. 2005. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 68:2298-2303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

