Activation of LXR increases acyl-CoA synthetase activity through direct regulation of ACSL3 in human placental trophoblast cells
- PMID: 20219900
- PMCID: PMC2882745
- DOI: 10.1194/jlr.M004978
Activation of LXR increases acyl-CoA synthetase activity through direct regulation of ACSL3 in human placental trophoblast cells
Abstract
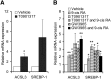
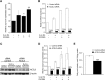
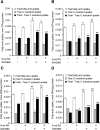
Placental fatty acid transport and metabolism are important for proper growth and development of the feto-placental unit. The nuclear receptors, liver X receptors alpha and beta (LXRalpha and LXRbeta), are key regulators of lipid metabolism in many tissues, but little is known about their role in fatty acid transport and metabolism in placenta. The current study investigates the LXR-mediated regulation of long-chain acyl-CoA synthetase 3 (ACSL3) and its functions in human placental trophoblast cells. We demonstrate that activation of LXR increases ACSL3 expression, acyl-CoA synthetase activity, and fatty acid uptake in human tropholast cells. Silencing of ACSL3 in these cells attenuates the LXR-mediated increase in acyl-CoA synthetase activity. Furthermore, we show that ACSL3 is directly regulated by LXR through a conserved LXR responsive element in the ACSL3 promoter. Our results suggest that LXR plays a regulatory role in fatty acid metabolism by direct regulation of ACSL3 in human placental trophoblast cells.
Figures


Similar articles
-
High-fructose diet downregulates long-chain acyl-CoA synthetase 3 expression in liver of hamsters via impairing LXR/RXR signaling pathway.J Lipid Res. 2013 May;54(5):1241-54. doi: 10.1194/jlr.M032599. Epub 2013 Feb 20. J Lipid Res. 2013. PMID: 23427282 Free PMC article.
-
The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia?Placenta. 2010 Oct;31(10):910-8. doi: 10.1016/j.placenta.2010.07.009. Epub 2010 Aug 14. Placenta. 2010. PMID: 20709391
-
The N-terminal region of acyl-CoA synthetase 3 is essential for both the localization on lipid droplets and the function in fatty acid uptake.J Lipid Res. 2012 May;53(5):888-900. doi: 10.1194/jlr.M024562. Epub 2012 Feb 22. J Lipid Res. 2012. PMID: 22357706 Free PMC article.
-
Fetal growth and development: roles of fatty acid transport proteins and nuclear transcription factors in human placenta.Indian J Exp Biol. 2004 Aug;42(8):747-57. Indian J Exp Biol. 2004. PMID: 15573522 Review.
-
Role of nuclear receptors and their ligands in human trophoblast invasion.J Reprod Immunol. 2008 Apr;77(2):161-70. doi: 10.1016/j.jri.2007.05.004. Epub 2007 Aug 15. J Reprod Immunol. 2008. PMID: 17706792 Review.
Cited by
-
Physiology and Pathophysiology of Steroid Biosynthesis, Transport and Metabolism in the Human Placenta.Front Pharmacol. 2018 Sep 12;9:1027. doi: 10.3389/fphar.2018.01027. eCollection 2018. Front Pharmacol. 2018. PMID: 30258364 Free PMC article. Review.
-
Liver X receptor regulates hepatic nuclear O-GlcNAc signaling and carbohydrate responsive element-binding protein activity.J Lipid Res. 2015 Apr;56(4):771-85. doi: 10.1194/jlr.M049130. Epub 2015 Feb 27. J Lipid Res. 2015. PMID: 25724563 Free PMC article.
-
LXRα Regulates ChREBPα Transactivity in a Target Gene-Specific Manner through an Agonist-Modulated LBD-LID Interaction.Cells. 2020 May 13;9(5):1214. doi: 10.3390/cells9051214. Cells. 2020. PMID: 32414201 Free PMC article.
-
ACSL3 is a promising therapeutic target for alleviating anxiety and depression in Alzheimer's disease.Geroscience. 2025 Apr;47(2):2383-2397. doi: 10.1007/s11357-024-01424-5. Epub 2024 Nov 13. Geroscience. 2025. PMID: 39532829 Free PMC article.
-
Maternal Vitamin and Mineral Supplementation and Rate of Maternal Weight Gain Affects Placental Expression of Energy Metabolism and Transport-Related Genes.Genes (Basel). 2021 Mar 9;12(3):385. doi: 10.3390/genes12030385. Genes (Basel). 2021. PMID: 33803164 Free PMC article.
References
-
- Fujino T., Kang M. J., Suzuki H., Iijima H., Yamamoto T. 1996. Molecular characterization and expression of rat acyl-CoA synthetase 3. J. Biol. Chem. 271: 16748–16752. - PubMed
-
- Hall A. M., Smith A. J., Bernlohr D. A. 2003. Characterization of the Acyl-CoA synthetase activity of purified murine fatty acid transport protein 1. J. Biol. Chem. 278: 43008–43013. - PubMed
-
- Hall A. M., Wiczer B. M., Herrmann T., Stremmel W., Bernlohr D. A. 2005. Enzymatic properties of purified murine fatty acid transport protein 4 and analysis of acyl-CoA synthetase activities in tissues from FATP4 null mice. J. Biol. Chem. 280: 11948–11954. - PubMed
-
- Oikawa E., Iijima H., Suzuki T., Sasano H., Sato H., Kamataki A., Nagura H., Kang M. J., Fujino T., Suzuki H., et al. 1998. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J. Biochem. 124: 679–685. - PubMed
-
- Pei Z., Oey N. A., Zuidervaart M. M., Jia Z., Li Y., Steinberg S. J., Smith K. D., Watkins P. A. 2003. The acyl-CoA synthetase “bubblegum” (lipidosin): further characterization and role in neuronal fatty acid beta-oxidation. J. Biol. Chem. 278: 47070–47078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

