B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease
- PMID: 20219916
- PMCID: PMC2863796
- DOI: 10.1128/JVI.02169-09
B cells and platelets harbor prion infectivity in the blood of deer infected with chronic wasting disease
Abstract
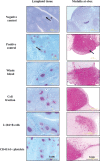
Substantial evidence for prion transmission via blood transfusion exists for many transmissible spongiform encephalopathy (TSE) diseases. Determining which cell phenotype(s) is responsible for trafficking infectivity has important implications for our understanding of the dissemination of prions, as well as their detection and elimination from blood products. We used bioassay studies of native white-tailed deer and transgenic cervidized mice to determine (i) if chronic wasting disease (CWD) blood infectivity is associated with the cellular versus the cell-free/plasma fraction of blood and (ii) in particular if B-cell (MAb 2-104(+)), platelet (CD41/61(+)), or CD14(+) monocyte blood cell phenotypes harbor infectious prions. All four deer transfused with the blood mononuclear cell fraction from CWD(+) donor deer became PrP(CWD) positive by 19 months postinoculation, whereas none of the four deer inoculated with cell-free plasma from the same source developed prion infection. All four of the deer injected with B cells and three of four deer receiving platelets from CWD(+) donor deer became PrP(CWD) positive in as little as 6 months postinoculation, whereas none of the four deer receiving blood CD14(+) monocytes developed evidence of CWD infection (immunohistochemistry and Western blot analysis) after 19 months of observation. Results of the Tg(CerPrP) mouse bioassays mirrored those of the native cervid host. These results indicate that CWD blood infectivity is cell associated and suggest a significant role for B cells and platelets in trafficking CWD infectivity in vivo and support earlier tissue-based studies associating putative follicular B cells with PrP(CWD). Localization of CWD infectivity with leukocyte subpopulations may aid in enhancing the sensitivity of blood-based diagnostic assays for CWD and other TSEs.
Figures
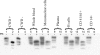

Similar articles
-
Alteration of the chronic wasting disease species barrier by in vitro prion amplification.J Virol. 2011 Sep;85(17):8528-37. doi: 10.1128/JVI.00809-11. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697475 Free PMC article.
-
Aerosol and nasal transmission of chronic wasting disease in cervidized mice.J Gen Virol. 2010 Jun;91(Pt 6):1651-8. doi: 10.1099/vir.0.017335-0. Epub 2010 Feb 17. J Gen Virol. 2010. PMID: 20164261 Free PMC article.
-
Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer.PLoS One. 2009 Nov 24;4(11):e7990. doi: 10.1371/journal.pone.0007990. PLoS One. 2009. PMID: 19956732 Free PMC article.
-
Chronic wasting disease of cervids.Curr Top Microbiol Immunol. 2004;284:193-214. doi: 10.1007/978-3-662-08441-0_8. Curr Top Microbiol Immunol. 2004. PMID: 15148993 Review.
-
Gene-Edited Cell Models to Study Chronic Wasting Disease.Viruses. 2022 Mar 15;14(3):609. doi: 10.3390/v14030609. Viruses. 2022. PMID: 35337016 Free PMC article. Review.
Cited by
-
Classical scrapie prions in ovine blood are associated with B lymphocytes and platelet-rich plasma.BMC Vet Res. 2011 Nov 23;7:75. doi: 10.1186/1746-6148-7-75. BMC Vet Res. 2011. PMID: 22112371 Free PMC article.
-
Preclinical detection of variant CJD and BSE prions in blood.PLoS Pathog. 2014 Jun 12;10(6):e1004202. doi: 10.1371/journal.ppat.1004202. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945656 Free PMC article.
-
New generation QuIC assays for prion seeding activity.Prion. 2012 Apr-Jun;6(2):147-52. doi: 10.4161/pri.19430. Epub 2012 Apr 1. Prion. 2012. PMID: 22421206 Free PMC article. Review.
-
Platelets in Neurodegenerative Conditions-Friend or Foe?Front Immunol. 2020 May 5;11:747. doi: 10.3389/fimmu.2020.00747. eCollection 2020. Front Immunol. 2020. PMID: 32431701 Free PMC article. Review.
-
Chronic Wasting Disease Transmission Risk Assessment for Farmed Cervids in Minnesota and Wisconsin.Viruses. 2021 Aug 11;13(8):1586. doi: 10.3390/v13081586. Viruses. 2021. PMID: 34452450 Free PMC article.
References
-
- Andréoletti, O., P. Berthon, E. Levavasseur, D. Marc, F. Lantier, E. Monks, J. M. Elsen, and F. Schelcher. 2002. Phenotyping of protein-prion (PrPsc)-accumulating cells in lymphoid and neural tissues of naturally scrapie-affected sheep by double-labeling immunohistochemistry. J. Histochem. Cytochem. 50:1357-1370. - PubMed
-
- Bons, N., S. Lehmann, N. Mestre-Frances, D. Dormant, and P. Brown. 2002. Brain and buffy coat transmission of bovine spongiform encephalopathy to the primate Microcebus murinus. Transfusion 42:513-516. - PubMed
-
- Brown, P. 1995. Can Creutzfeldt-Jakob disease be transmitted by transfusion? Curr. Opin. Hematol. 2:472-477. - PubMed
-
- Brown, P., L. Cervenakova, P. McShane, B. R. Rubenstein, and W. N. Drohan. 1999. Further studies of blood infectivity in an experimental model of transmissible spongiform encephalopathy, with an explanation of why blood components do not transmit Creutzfeldt-Jakob disease in humans. Transfusion 39:1169-1178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

