Early spatial and temporal events of human T-lymphotropic virus type 1 spread following blood-borne transmission in a rabbit model of infection
- PMID: 20219918
- PMCID: PMC2863820
- DOI: 10.1128/JVI.01537-09
Early spatial and temporal events of human T-lymphotropic virus type 1 spread following blood-borne transmission in a rabbit model of infection
Abstract
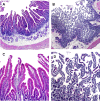
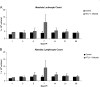
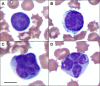
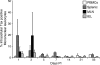
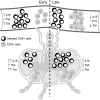
Human T-lymphotropic virus type 1 (HTLV-1) infection causes adult T-cell leukemia/lymphoma (ATL) and is associated with a variety of lymphocyte-mediated disorders. HTLV-1 transmission occurs by transmission of infected cells via breast-feeding by infected mothers, sexual intercourse, and contaminated blood products. The route of exposure and early virus replication events are believed to be key determinants of virus-associated spread, antiviral immune responses, and ultimately disease outcomes. The lack of knowledge of early events of HTLV-1 spread following blood-borne transmission of the virus in vivo hinders a more complete understanding of the immunopathogenesis of HTLV-1 infections. Herein, we have used an established animal model of HTLV-1 infection to study early spatial and temporal events of the viral infection. Twelve-week-old rabbits were injected intravenously with cell-associated HTLV-1 (ACH-transformed R49). Blood and tissues were collected at defined intervals throughout the study to test the early spread of the infection. Antibody and hematologic responses were monitored throughout the infection. HTLV-1 intracellular Tax and soluble p19 matrix were tested from ex vivo cultured lymphocytes. Proviral copy numbers were measured by real-time PCR from blood and tissue mononuclear leukocytes. Our data indicate that intravenous infection with cell-associated HTLV-1 targets lymphocytes located in both primary lymphoid and gut-associated lymphoid compartments. A transient lymphocytosis that correlated with peak virus detection parameters was observed by 1 week postinfection before returning to baseline levels. Our data support emerging evidence that HTLV-1 promotes lymphocyte proliferation preceding early viral spread in lymphoid compartments to establish and maintain persistent infection.
Figures
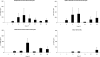


References
-
- Anderson, D. C., J. Epstein, L. Pierik, J. Solomon, W. Blattner, C. Saxinger, H. Alter, H. Klein, P. McCurdy, G. Nemo, J. Kaplan, J. Allen, R. Khabbaz, and M. Lairmore. 1988. Licensure of screening tests for antibody to human T-cell lymphotropic virus type I. MMWR Morb. Mortal. Wkly. Rep. 37:736. - PubMed
-
- Asquith, B., A. J. Mosley, A. Barfield, S. E. Marshall, A. Heaps, P. Goon, E. Hanon, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2005. A functional CD8+ cell assay reveals individual variation in CD8+ cell antiviral efficacy and explains differences in human T-lymphotropic virus type 1 proviral load. J. Gen. Virol. 86:1515-1523. - PubMed
-
- Bangham, C. R., K. Meekings, F. Toulza, M. Nejmeddine, E. Majorovits, B. Asquith, and G. P. Taylor. 2009. The immune control of HTLV-1 infection: selection forces and dynamics. Front. Biosci. 14:2889-2903. - PubMed
-
- De, B. K., M. D. Lairmore, K. Griffis, L. J. Williams, F. Villinger, T. C. Quinn, C. Brown, E. Nzilambi, M. Sugimoto, and S. Araki. 1991. Comparative analysis of nucleotide sequences of the partial envelope gene (5′ domain) among human T lymphotropic virus type I (HTLV-I) isolates. Virology 182:413-419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

