Jaagsiekte sheep retrovirus transformation in Madin-Darby canine kidney epithelial cell three-dimensional culture
- PMID: 20219922
- PMCID: PMC2863794
- DOI: 10.1128/JVI.02323-09
Jaagsiekte sheep retrovirus transformation in Madin-Darby canine kidney epithelial cell three-dimensional culture
Abstract
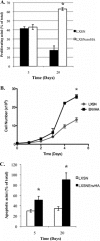
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of a contagious lung cancer in sheep that shares similarities with human bronchioloalveolar carcinoma (BAC). JSRV is unique because the envelope gene (env) is the oncogene, as it can transform cells in culture and induce tumors in animals. The phosphatidylinositol 3-kinase (PI3K)-Akt-mTOR and H/N-Ras-MEK-mitogen-activated protein kinase (MAPK) pathways have been shown to be critical for Env transformation. However, the question still remains of how disruption of these pathways relates to tumor formation. To address this, JSRV Env transformation was studied in the context of epithelial structure, using the polarized Madin-Darby canine kidney (MDCK) epithelial cell three-dimensional (3-D) culture system. The results indicated that JSRV Env-transformed MDCK cells were larger and had full or multiple lumens, in contrast to the single lumens observed in controls. The altered phenotype was largely mediated by an increase in proliferation, in addition to overcoming the proliferative suppression signal. JSRV Env was not found to disrupt polarity or tight junctions or to inhibit lumen apoptosis. The PI3K-Akt-mTOR pathway was important for Env transformation in MDCK cells, although the mechanisms of action differed in 3-D and monolayer cultures. PI3K-dependent signaling to mTOR occurred in monolayers, while PI3K-independent signaling to mTOR occurred in 3-D culture. In contrast, the H/N-Ras-MEK-MAPK pathway was found to be inhibitory to transformation in both normal and transformed MDCK cells in 3-D culture. However, in monolayer culture, inhibition of MEK reverted the transformed phenotype, suggesting a different mechanism(s) of action in monolayer versus 3-D culture.
Figures
Similar articles
-
Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines.J Virol. 2005 Apr;79(7):4440-50. doi: 10.1128/JVI.79.7.4440-4450.2005. J Virol. 2005. PMID: 15767444 Free PMC article.
-
Transformation of madin-darby canine kidney epithelial cells by sheep retrovirus envelope proteins.J Virol. 2005 Jan;79(2):927-33. doi: 10.1128/JVI.79.2.927-933.2005. J Virol. 2005. PMID: 15613321 Free PMC article.
-
Putative phosphatidylinositol 3-kinase (PI3K) binding motifs in ovine betaretrovirus Env proteins are not essential for rodent fibroblast transformation and PI3K/Akt activation.J Virol. 2003 Jul;77(14):7924-35. doi: 10.1128/jvi.77.14.7924-7935.2003. J Virol. 2003. PMID: 12829832 Free PMC article.
-
Oncogenic transformation by the jaagsiekte sheep retrovirus envelope protein.Oncogene. 2007 Feb 8;26(6):789-801. doi: 10.1038/sj.onc.1209850. Epub 2006 Aug 14. Oncogene. 2007. PMID: 16909114 Review.
-
Jaagsiekte Sheep Retrovirus (JSRV): from virus to lung cancer in sheep.Vet Res. 2007 Mar-Apr;38(2):211-28. doi: 10.1051/vetres:2006060. Epub 2007 Jan 25. Vet Res. 2007. PMID: 17257570 Review.
Cited by
-
E7 oncoprotein from human papillomavirus 16 alters claudins expression and the sealing of epithelial tight junctions.Int J Oncol. 2020 Oct;57(4):905-924. doi: 10.3892/ijo.2020.5105. Epub 2020 Jul 29. Int J Oncol. 2020. PMID: 32945372 Free PMC article.
-
Enhanced proliferation of primary rat type II pneumocytes by Jaagsiekte sheep retrovirus envelope protein.Virology. 2011 Apr 10;412(2):349-56. doi: 10.1016/j.virol.2011.01.022. Epub 2011 Feb 12. Virology. 2011. PMID: 21316726 Free PMC article.
-
SAMHD1 inhibits epithelial cell transformation in vitro and affects leukemia development in xenograft mice.Cell Cycle. 2018;17(23):2564-2576. doi: 10.1080/15384101.2018.1550955. Epub 2018 Dec 4. Cell Cycle. 2018. PMID: 30474474 Free PMC article.
-
Lung adenocarcinoma originates from retrovirus infection of proliferating type 2 pneumocytes during pulmonary post-natal development or tissue repair.PLoS Pathog. 2011 Mar;7(3):e1002014. doi: 10.1371/journal.ppat.1002014. Epub 2011 Mar 31. PLoS Pathog. 2011. PMID: 21483485 Free PMC article.
-
Early Steps of Jaagsiekte Sheep Retrovirus-Mediated Cell Transformation Involve the Interaction between Env and the RALBP1 Cellular Protein.J Virol. 2015 Aug;89(16):8462-73. doi: 10.1128/JVI.00590-15. Epub 2015 Jun 3. J Virol. 2015. PMID: 26041289 Free PMC article.
References
-
- Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The Jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

