Complex interactions between the major and minor envelope proteins of equine arteritis virus determine its tropism for equine CD3+ T lymphocytes and CD14+ monocytes
- PMID: 20219931
- PMCID: PMC2863813
- DOI: 10.1128/JVI.02743-09
Complex interactions between the major and minor envelope proteins of equine arteritis virus determine its tropism for equine CD3+ T lymphocytes and CD14+ monocytes
Abstract
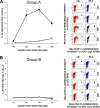
Extensive cell culture passage of the virulent Bucyrus (VB) strain of equine arteritis virus (EAV) to produce the modified live virus (MLV) vaccine strain has altered its tropism for equine CD3(+) T lymphocytes and CD14(+) monocytes. The VB strain primarily infects CD14(+) monocytes and a small subpopulation of CD3(+) T lymphocytes (predominantly CD4(+) T lymphocytes), as determined by dual-color flow cytometry. In contrast, the MLV vaccine strain has a significantly reduced ability to infect CD14(+) monocytes and has lost its capability to infect CD3(+) T lymphocytes. Using a panel of five recombinant chimeric viruses, we demonstrated that interactions among the GP2, GP3, GP4, GP5, and M envelope proteins play a major role in determining the CD14(+) monocyte tropism while the tropism for CD3(+) T lymphocytes is determined by the GP2, GP4, GP5, and M envelope proteins but not the GP3 protein. The data clearly suggest that there are intricate interactions among these envelope proteins that affect the binding of EAV to different cell receptors on CD3(+) T lymphocytes and CD14(+) monocytes. This study shows, for the first time, that CD3(+) T lymphocytes may play an important role in the pathogenesis of equine viral arteritis when horses are infected with the virulent strains of EAV.
Figures



Similar articles
-
Equine arteritis virus.Vet Microbiol. 2013 Nov 29;167(1-2):93-122. doi: 10.1016/j.vetmic.2013.06.015. Epub 2013 Jul 3. Vet Microbiol. 2013. PMID: 23891306 Free PMC article. Review.
-
Experiences with infectious cDNA clones of equine arteritis virus: lessons learned and insights gained.Virology. 2014 Aug;462-463:388-403. doi: 10.1016/j.virol.2014.04.029. Epub 2014 Jun 7. Virology. 2014. PMID: 24913633 Free PMC article. Review.
-
Arterivirus minor envelope proteins are a major determinant of viral tropism in cell culture.J Virol. 2012 Apr;86(7):3701-12. doi: 10.1128/JVI.06836-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258262 Free PMC article.
-
Chimeric viruses containing the N-terminal ectodomains of GP5 and M proteins of porcine reproductive and respiratory syndrome virus do not change the cellular tropism of equine arteritis virus.Virology. 2012 Oct 10;432(1):99-109. doi: 10.1016/j.virol.2012.05.022. Epub 2012 Jun 26. Virology. 2012. PMID: 22739441
-
Equine Arteritis Virus Has Specific Tropism for Stromal Cells and CD8+ T and CD21+ B Lymphocytes but Not for Glandular Epithelium at the Primary Site of Persistent Infection in the Stallion Reproductive Tract.J Virol. 2017 Jun 9;91(13):e00418-17. doi: 10.1128/JVI.00418-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28424285 Free PMC article.
Cited by
-
Development and use of a polarized equine upper respiratory tract mucosal explant system to study the early phase of pathogenesis of a European strain of equine arteritis virus.Vet Res. 2013 Mar 28;44(1):22. doi: 10.1186/1297-9716-44-22. Vet Res. 2013. PMID: 23537375 Free PMC article.
-
Equine arteritis virus long-term persistence is orchestrated by CD8+ T lymphocyte transcription factors, inhibitory receptors, and the CXCL16/CXCR6 axis.PLoS Pathog. 2019 Jul 29;15(7):e1007950. doi: 10.1371/journal.ppat.1007950. eCollection 2019 Jul. PLoS Pathog. 2019. PMID: 31356622 Free PMC article.
-
Equine arteritis virus.Vet Microbiol. 2013 Nov 29;167(1-2):93-122. doi: 10.1016/j.vetmic.2013.06.015. Epub 2013 Jul 3. Vet Microbiol. 2013. PMID: 23891306 Free PMC article. Review.
-
Experiences with infectious cDNA clones of equine arteritis virus: lessons learned and insights gained.Virology. 2014 Aug;462-463:388-403. doi: 10.1016/j.virol.2014.04.029. Epub 2014 Jun 7. Virology. 2014. PMID: 24913633 Free PMC article. Review.
-
Intrahost Selection Pressure Drives Equine Arteritis Virus Evolution during Persistent Infection in the Stallion Reproductive Tract.J Virol. 2019 May 29;93(12):e00045-19. doi: 10.1128/JVI.00045-19. Print 2019 Jun 15. J Virol. 2019. PMID: 30918077 Free PMC article.
References
-
- Balasuriya, U. B., J. F. Evermann, J. F. Hedges, A. J. McKeirnan, J. Q. Mitten, J. C. Beyer, W. H. McCollum, P. J. Timoney, and N. J. MacLachlan. 1998. Serologic and molecular characterization of an abortigenic strain of equine arteritis virus isolated from infective frozen semen and an aborted equine fetus. J. Am. Vet. Med. Assoc. 213:1586-1589. - PubMed
-
- Balasuriya, U. B., H. W. Heidner, N. L. Davis, H. M. Wagner, P. J. Hullinger, J. F. Hedges, J. C. Williams, R. E. Johnston, W. David Wilson, I. K. Liu, and N. James MacLachlan. 2002. Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20:1609-1617. - PubMed
-
- Balasuriya, U. B., C. M. Leutenegger, J. B. Topol, W. H. McCollum, P. J. Timoney, and N. J. MacLachlan. 2002. Detection of equine arteritis virus by real-time TaqMan reverse transcription-PCR assay. J. Virol. Methods 101:21-28. - PubMed
-
- Balasuriya, U. B., and N. J. MacLachlan. 2004. The immune response to equine arteritis virus: potential lessons for other arteriviruses. Vet. Immunol. Immunopathol. 102:107-129. - PubMed
-
- Balasuriya, U. B., E. J. Snijder, H. W. Heidner, J. Zhang, J. C. Zevenhoven-Dobbe, J. D. Boone, W. H. McCollum, P. J. Timoney, and N. J. Maclachlan. 2007. Development and characterization of an infectious cDNA clone of the virulent Bucyrus strain of equine arteritis virus. J. Gen. Virol. 88:918-924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

